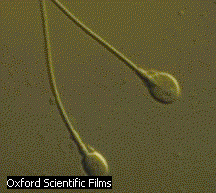
 sperm
sperm
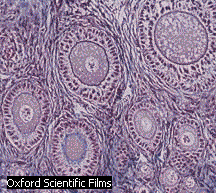 eggs
eggsCells - A Primer
Humans are composed of about 100 trillion cells, with the brain alone having over 100 billion cels - close to the number of starts in the Milky Way galaxy. Just think - all that starts from 1 cell - a fertilized egg. The egg and sperm, also known as germ cells, contain half the number of chromosomes (23) as in a body cell (23x2), also called a somatic cell. To a first approximation, all the somatic cells have the same DNA. Hence you could theoretically clone an individual from a single somatic cell since it contains all 23 pairs of chromosomes- the full genetic blueprint. You have heard about many different kinds of body cells, some of which are shown below. Of the body cells shown, the red blood cells are a bit unusual. They don't have a nucleus and hence have no DNA!. The are made, however, from another kind of cell that does have DNA.
 sperm
sperm
 eggs
eggs
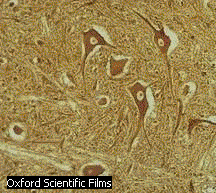 nerve cell
nerve cell  cardiac cells
cardiac cells
 muscle
muscle
 red
blood cell
red
blood cell
A question should immediately pop to your head. How could so many different kinds of cells be produced from one cell - the fertilized egg, especially since they all have the same DNA. The body cells shown are clearly different from each other and from the starting fertilized egg. Somehow, the cells have differentiated from the fertilized egg in the process of development. The fertilized egg is considered to be a totipotent stem cell, since it has the potential to become, through division and differentiation, any kind of cell in the body.
The differentiated cells in the body are different not because their DNA is different, but because the DNA blueprint has been read out differently in each cell type. Remember there are about 30-40 thousand different genes in the human genome. Not all of the genes are read out or expressed (often called turned on) - i.e. transcribed into mRNA and the mRNA translated into protein - in any given cell. Muscle cells and neurons differ in that different genes have been turned off and turned on at different times in the development of the cells. Hence the cells, with the same DNA, are composed of different proteins which make the cells look different and have different functions. What controls this process of turning genes on and off?
Genes Expression: Development
Imagine a fertilized cell. It divides into two daughter cells, which divide into 4 cells, which divide into 8 cells, ..... Are all the resulting cells the same? Have any of the cells become different from the original fertilized egg? (i.e. have any of the cells differentiated?). It turns out that at the 8 cell stage, the developing zygote consists of identical cells. Any one of the cells can be removed and the remaining 7 after insertion into the uterus can lead to the development of a healthy baby. This may seem like a strange experiment, but it has been done for couples that carrier a high risk of passing on lethal genetic traits (like Tay-Sachs Disease). The DNA from the 1 removed egg can be analyzed to determine if the cell contains the deleterious gene.
At some point in the process of development of the zygote, the cells do differentiate, and ultimately become liver, skeletal, muscle, neural, blood cells, etc. What happens to cause them to differentiate? At some point, it must come down to the environment of the cells. If you image a large ball of cells, some of them will be on the outside and some on the inside of the ball. They now are clearly in different microenvironments. Somehow the cells sense they are, and these different environmental signals cause the cells to differentiate. The cells can sense their outer environment, which signals the inside of the cell to turn on or off certain genes, leading cells down the path of differentiation. This process of a cells responding to an external environmental cue is call signal transduction.
The DNA of humans and our closest evolutionary relative, the chimpanzee, is 98.7% identical. Most of our genes are identical. How can we be so dissimilar? How does the dissimilarity arise? Paabo et al (Science, 296, pg 340, 2002) compared the RNA expressed from 12,000 different genes from different organs (brain, liver, blood) from humans, chimps, macaques, and orangutans. There were little differences in expression of genes in liver and blood. The only big difference was between expression of genes in human brain compared to the rest of the primates.
Gene Expression: Signal Transduction
Cells have to respond to their environment all the time. Immune cells have to respond to the presence of a tumor cell, a bacteria or virus. Retinal cells have to respond to light. Many cells respond to hormones - chemical signals that circulate in the blood. Neurons respond to neurotransmitters - molecules released by adjacent neurons. The responses of the cell can be many: the cell could divide, stop dividing, kill itself, secrete a hormone, take up some nutrients like sugar or vitamins, fire a nerve signal, etc. Problems with the signal transduction can cause big trouble. A cell that should live a normal life span or actually kill itself might live forever and become a tumor cell. A cell that should live might die, which might lead to the manifestations of autoimmune disease (when the body attacks self by mistake) or neurodegenerative diseae (like Alzheimers, Lou Gehrigs Disease, Parkinson Disease, etc.).
Tumor cells (characterized by unregulated cell growth and cell immortaliity) are clearly different from normal cells (characterized by regulated growth and a finite cell life). Somehow the normal signaling machinery which puts the brakes on cell growth and division has gone wrong. Normal cells can become tumor cells after enough critical genes have undergone mutations, or in some cases, if the cell is infected with certain viruses that are known to promote cancer. The tumor cell, since it has different properties than the normal cell, must be making different protein than the normal cell (in addition to the proteins that may have been altered through mutation or viral infection).
New ways have been recently developed to analyse which genes are turned on and off in diseased cells (like tumor cells) compared to normal cells. These involve a system using gene arrays.
DNA Binding and Genomic Analyses: Gene Expression in Disease
Large numbers (100,000 to 1 million) of different DNA molecules can be chemically attached to silicon or glass chips. These sequences are located at specific x,y coordinates on the chip. DNA or mRNA probes that will bind to the DNA on the chips can be made from cells. A chemical group can be attached to the probes to make them fluorescent. When added to the chip, they will bind through complementary intermolecular interactions to specific DNA's on the chip. Using this technique, it is possible to determine which genes are expressed in a given cell and at a given time in a given individual. In addition, with the right DNA molecules on the array, it will be possible to check for polymorphisms or differences in genes between individuals. In this way it will be possible to determine if a person has a particular mutation in a given gene that might make them susceptible to a given disease.
In a specific example, mRNA can be extracted from two different cells, a control and a tumor cell. The control mRNA can be labeled with a green fluorescent molecule, while the tumor cell mRNA can be labeled with a red tag. They can both be added to the chip containing a "library" of human genes. If the gene is expressed in both cell types, both types of labeled mRNA will bind and the spot on the chip will appear yellow. If the gene is not expressed in either tissue, the spot will appear black. Genes that are only expressed in tumor cells will appear red and in control cells green. In a single experiment, the differential expression of genes in tumor cells can be determined. In this way, tumor-specific proteins can be identified, which could lead to the development of a vaccine against those tumor antigens. A typical microarray analyis for this type of experiment is shown below. (from Nature, 403, 699 (2000).
Detection of Differential Gene Expression in Tumors and Normal Cells Using Microarray Chip
Another example of the power of microarrays is given below.
"Human breast tumours are diverse in their natural history and in their responsiveness to treatments. Variation in transcriptional regulation accounts for much of the biological diversity of human cells and tumours. In each cell, signal transduction and regulatory systems transduce information between the cell's identity and its environmental status, thereby controlling the level of expression of every gene in the genome. Here we have characterized variation in gene expression patterns in a set of 65 surgical specimens of human breast tumours from 42 different individuals, using complementary DNA microarrays representing 8,102 human genes. These patterns provided a distinctive molecular portrait of each tumour. Twenty of the tumours were sampled twice, before and after a 16-week course of chemotherapy, and two tumours were paired with a lymph node metastasis from the same patient. Gene expression patterns in two tumour samples from the same individual were almost always more similar to each other than either was to any other sample. Sets of co-expressed genes were identified for which variation in messenger RNA levels could be related to specific features of physiological variation. The tumours could be classified into subtypes distinguished by pervasive differences in their gene expression patterns.
Variation in the expression of 1753 genes in 84 experimental samples.
Caption for figure: Variation in expression of 1,753 genes in 84 experimental samples. Data are presented in a matrix format: each row represents a single gene, and each column an experimental sample. In each sample, the ratio of the abundance of transcripts of each gene to the median abundance of the gene's transcript among all the cell lines (left panel), or to its median abundance across all tissue samples (right panel), is represented by the colour of the corresponding cell in the matrix. Green squares, transcript levels below the median; black squares, transcript levels equal to the median; red squares, transcript levels greater than the median; grey squares, technically inadequate or missing data. Colour saturation reflects the magnitude of the ratio relative to the median for each set of samples (see scale, bottom left)." Nature 406, 747 - 752 (2000) © Macmillan Publishers Ltd.
DNA arrays will prove extremely powerful in diagnosis and treatment of diseases. In the above example, it can be used to differentiate tumor more susceptible to chemotherapy, for example.
New Medical Therapies
For dieases other than infectious diseases (caused by viruses and bacteria), the traditional method of treatment has been drug therapy. For example, diabetics are treated with insulin, people with cardivascular disease are often given statins to reduce their cholesterol and drugs to reduce blood pressure. These drugs invariably work by binding to specific proteins, often enzyme catalyst, to block their activity. Often, however, these drugs just treat the symptoms and not the cause of the disease.
Wouldn't it be bettter to be able to give diabetes a new gene to make insulin to replace their faculty gene (if that is what caused their diabetes). This is an example of the gene therapy, a field still in its infancy. Alternatively, wouldn't it be better to give a diabetic new pancreatic islet cells, replacing the ones destroyed during their disease? Or give to a patient with Parkinsons disease cells that could make the neurotransmiter dopamine?
Stem Cells
As mentioned earlier, the fertilized egg is a totipotent stem cell, since all other cells in the body stem from it. There are other kinds of pluripotent stem cells that are not quite totipotent but still can differentiate into a variety of other cell types. It would make sense that these might be very useful in cell replacement therapy. They could be given to individuals, for instance, with a neurodegenerative brain disease. The stem cells, under the environmental conditions of the brain, might then differentiate into dopamine-containing neurons or another type of brain cell - a glial cell - which facilitates neuron functioning. How about treating the devasting effects of multiple sclerosis or muscular dystrophy? Maybe pluripotent stem cells could treat the effects of aging or trauma (like a spinal cord injury which leads to paralysis). The technical definition of a stem cells are cells with the capacity for unlimited or prolonged self-renewal that can produce at least one type of highly differentiated descendant.
Research has shown that some stem cells are pluripotent: and can differentiate into
many different cell type. but stem cells of certain tissues, such as epithelial
structures, can work only in curtain cellular environments. It appears that stem
cells can be found in many tissue, such as in the adult central nervous system and
bone marrow, that could be used to replace blood and immune cells. These type
of cells are called adult stem cells since they derive from adult tissue.
It should also be apparent that younger cells, closer to the original totipotent stem
cells of the fertilized egg, would be even better stem cells. Stem cells can be
derived from an embryo (obtained from spare embryos from in vitro fertilzation procedures)
or fetus germ cells (obtained from abortions). In vertebrates, the
zygote is a totipotent stem cell, as are virtually all of its progeny around the blastula
stage; cells contained within the inner cell mass (ICM), include (and may be composed of)
totipotent stem cells. Embryonic stem (ES) cells are derived from
laboratory cultures of ICM cells, and have the property of participating as totipotent
cells when placed into host blastocysts." Clearly use of these cells
poses moral questions which need to be addressed.
American Association for the Advancement of Science: Report on Stem Cells
National Institute of Health: Stem Cell Homepage
Stem Cell Primer from the NIH
A normal human cell can be changed into a tumor cell.
Changing Gene Expression: Genetic Therapy in Somatic Cells
If a disease is caused by a defective gene, why not just replace the gene. Gene Therapy holds such promise. Many problems must be resolved before gene therapy is widely use and reaches its promise. First of all you have to get the gene into some target cell, somehow it must be inserted into a chromosome in the target cell (so it will be replicated when the cell divides), it must be expressed at the right time and in the right amounts and for the correct length of time. The protein product of the gene might be seen as foreign by the body and be target for elimination by the immune system. Given all these problems, it is amazing that progress has actually been made. One reason is that nature has already give us sources of DNA that can enter specific cells and insert into the cells genetic material. The souce of the DNA is a virus, which, for part of its life cycle, can insert into host DNA. Viruses can be genetically engineered to insert a human gene of choice. The virus is then added to the cells, which hopefully take it up and ultimately express the human gene after the virus has inserted into the host DNA. Several different viruses which can act as vectors to shuttle a human gene into cells are described below.
"Retroviruses. The vector used to successfully treat SCID
(severe combined immunodifficiency) children in France was derived from the Moloney
retrovirus, an RNA virus that infects mice. Because it inserts its genes into the host's
genome, any genes artificially added to the vector are expressed for a long period. It is
efficient and seems to produce no strong immune response, but it only works in cells that
are actively dividing. Its other main disadvantage is that it integrates into DNA
randomly. Gene therapists say there is a remote but real chance that if a retrovirus
landed in the wrong location, it might promote cancer.
Adenovirus. Many vectors based on this "cannonball with
spikes," as one expert calls it, are being developed for gene therapy. A common DNA
virus that infects the human respiratory tract and eyes, it was the basis for the vector
used to treat a liver enzyme deficiency at the University of Pennsylvania (in which the
patient died from adenovirus-associated complications). Adenovirus is easy to grow in the
lab, and it readily infects both dividing and nondividing cells, expressing genes without
inserting itself into the host cell's genome or posing a risk of cancer. But adenovirus
proteins stimulate strong immune reactions that clear the vector from the body, making it
ineffective for long-term therapy. High doses may be required to transfer enough genes to
produce a health benefit. But high doses also can producepowerful toxic reactions when
given intravenously, as Penn's researchers discovered.
Adeno-associated virus (AAV). This parvovirus is nearly invisible to the
human immune system and readily infects human dividing and nondividing cells. It requires
a "helper," adenovirus, to replicate, and when it integrates into host DNA it
does so at a known and apparently safe location. It has some disadvantages: It's more
difficult to grow to high concentrations than adenovirus, and it has a small genome,
restricting the amount of therapeutic DNA it can carry. Researchers Mark Kay of Stanford
University and Katherine High of the Children's Hospital of Philadelphia recently used
this vector to transfer genes for a blood clotting protein (factor IX) into patients with
hemophilia B. Two patients in the first cohort of a safety trial appeared to improve, and
dogs lacking factor IX have shown benefits for as long as 2 years. In addition, Kay has
developed a technique
that may double AAV's gene-carrying capacity.
Lentiviruses. These slow-growing retroviruses are promising candidates
for vectors, according to one champion, gene therapy researcher Inder Verma of the Salk
Institute in La Jolla, California. He likes their "unique advantage of introducing
genes into dividing and nondividing cells" and their ability to survive without
producing a strong immune reaction in the host. The AIDS virus belongs to this family. And
despite its fearful origins, Verma is convinced that HIV can be tamed to create a useful
vector for gene therapy, although clinical trials may be a long way off. Herpes simplex
virus is another candidate in this family, prized because it can infect nervous system
cells, which are resistant to other vectors." Volume 288, Number 5468, Issue of 12
May 2000, p. 953. Copyright © 2000 by The American Association for the Advancement of
Science.
Some genetic diseases can result in great damage or death to fetuses before they are born. Hence scientist are now discussing the possibiilty of in utero gene therapy (IUGT).
Changing Gene Expression: Gene Therapy in Germ Cells
If gene therapy or modification is done on human germ cells, (i.e. sperm or egg), then the modifcation becomes inheritable. This is in start contrast to gene therapy or modification of somatic (body) cells, which can not be passed onto further progeny unless the modified cell is cloned. Imagine the possibilities of germ-line gene therapy! Imagine the moral issues!
NIH Report on Human Inheritable Genetic Modifications
Changing Gene Expression: Chemical Genetics
DNA can exist as single, double-stranded, or mixed forms. It is actually a misnomer to call dsDNA a molecule, since it really consisted of two different, complementary strands held together by IMF's. However, m
Pharmacogenetics
"Every individual is a product of the interaction of their genes and the environment. Pharmacogenetics is the study of how genetic differences influence the variability in patients' responses to drugs. Through the use of pharmacogenetics, we will soon be able to profile variations between individuals' DNA to predict responses to a particular medicine. The medical significance and economic value of a simple, predictive medicine response profile, which will provide information on the likelihood of efficacy and safety of a drug for an individual patient, will change the practice and economics of medicine. The ability to rapidly profile patients who are likely to benefit from a particular medicine will also streamline drug development and provide opportunities to develop discrete medicines concurrently for different patients with similar disease phenotypes. Other than relatively rare and highly penetrant diseases related to mutations of a single gene inherited in families, science has never before had the tools to characterize the nuances of inherited metabolic variations that interact over time and lead to common diseases. Powerful pharmacogenetic research tools are now becoming available to classify the heterogeneity of disease as well as individual responses to medicines....Pharmacogenetics is not gene therapy, not genetically modified foods, not genetic engineering, and not cloning of humans or their organs....
When we go to see our doctor, our symptoms and physical signs are
evaluated, and appropriate tests (for example, blood, urine, X-ray and magnetic resonance
imaging) are undertaken. To the non-physician, this process of disease diagnosis seems
straightforward. However, for a patient to have all the classical symptoms and signs of a
particular disease is the exception rather than the rule. How these diagnoses relate to
the underlying mechanism of disease is often unknown. For example, patients with mutations
in different genes may present as clinically identical. Mutations of APP, presenilin 1 and
presenilin 2 lead to clinically indistinguishable forms of Alzheimer's disease. It is also
important to note that mutations at different sites along the APP gene can lead to two
distinct diseases, early-onset Alzheimer's disease and recurrent intracerebral
haemorrhages. For many common diseases, the situation may be assumed to be even more
complicated, with many contributing molecular variants of several interacting
susceptibility genes leading to multiple clinical effects over varying time frames. Thus
many of the diseases that we classify clinically may be syndromes with several distinct
contributing pathogenic mechanisms. With all this clinical and genetic heterogeneity we
should not lose sight of the fact that the major objective is to treat, cure or prevent
disease. It is significant that a medicine works; does it matter whether it is effective
in patients who may have different diagnoses? The goal of medicine is to relieve pain and
suffering. Similar mechanisms may exist for quite diverse clinical diseases. As the
targets and mechanisms are validated in humans, additional clinical indications may become
more obvious because of shared mechanisms rather than similar clinical presentations.
How does your doctor know when making the diagnosis that medicines that are effective for
you have not been precluded? Pharmacogenetics will enable individuals to be classified
according to their likely response to a medicine. This is not a new concept as clinical
subtypes are often classified by drug responsiveness (for example, steroid-sensitive and
steroid-resistant asthma). Application of pharmacogenetics will expand the population to
those who can be helped but might have otherwise been missed because their clinical
syndrome did not fit neatly into a traditional disease category. Alosetron is a recently
approved medicine in the United States for the treatment of female patients with
diarrhoea-predominant irritable bowel syndrome (IBS). Most physicians will acknowledge
that the diagnosis of IBS can be imprecise — in fact, the 'disease' is truly a
syndrome. The value of a diagnostic test to sub-classify IBS into different types may be
limited, but a simple medicine response profile to determine whether the patient's
symptoms will be alleviated by alosetron could have considerable value. Pharmacogenetic
approaches will no doubt confirm what clinicians already know — disease diagnosis is
not easy nor necessarily homogeneous and accurate.
Apparently distinct diseases may have similar underlying mechanisms. A medicine developed
for a specific indication could have value in treating other related or non-related
conditions. This is also not a new concept. There are many medicines that were initially
registered with a single indication, which have then been expanded as more clinical
research is conducted. For example, carbamazepine was initially registered as a treatment
for trigeminal neuralgia, a syndrome with intermittent severe lightning-like bursts of
facial pain, but was later extended to treat various forms of epilepsy. By understanding
the genetic basis of patient responses to medicines, and perhaps also by having a better
understanding of how the medicine works, we will be able to identify additional clinical
indications more quickly.
Treatment How does a physician know if the medicine and the dose prescribed will be
effective and whether or not the patient will experience adverse effects? Information is
available from clinical trials in the medicine's data sheet/label in which similar
patients were included and the physician may use experience of treating previous patients.
On many occasions, the prescribed medicine will be effective and not cause serious side
effects. Other patients may not respond or suffer adverse reactions. By applying the
results of pharmacogenetic research to clinical practice, physicians will be able to use
information from patients' DNA to determine how patients are likely to respond to a
particular medicine. The clinical fact that the drug dose for some patients must be
individualized has been accepted for years. Polymorphisms in genes encoding P450 enzymes,
N-acetyltransferase and other key enzymes in drug metabolism account for the concentration
variation of certain drugs in patients' blood. It is also well established that some
patients can be slow in activating drugs and respond inadequately to some prodrugs, or
exhibit reduced clearance and increased effects from some pharmacologically active agents.
Enzyme tests that measure those variants have, in some cases, already been replaced with
genetic variants on chips. In the future, metabolic screens of genetic variants will be
standardized so that automated read-outs of each person's predicted response to each
medicine could be generated. These DNA-based screens will not provide disease-specific
diagnosis, but useful information to aid in individual dosing of medications or avoidance
of side effects." ALLEN D. ROSES, Nature 405, 857 - 865 (2000) © Macmillan
Publishers Ltd.
Pharmacogenetics and Its Impact on Drug Development from the Scientist.
Pharmacogenomics: Today, Tomorrow, and Beyond from Medline.
The new word in designer drugs from the British Medical Journal
PERSONAL PILLS: Genetic differences may dictate how drugs are prescribed from Scientific American
Pharmacogenomics and the future of medicine in GeneLetter
Changing Gene Expression: Cloning
"Cloning techniques have been in use for centuries. The
practice of taking cuttings is universal among gardeners, and large companies now
propagate desirable plant strains in their millions. Lower invertebrates can also be
cloned — cut an earthworm or flatworm in half, for example, and the missing halves
will regenerate to create two genetically identical individuals. Although vertebrates
cannot be cloned by these routes, identical twins are naturally occurring genetic clones.
Moreover, the method of nuclear transplantation, first developed about 40 years ago in
frogs, has been successfully used to make clones of sheep, mice, cows and goats, and it
could probably be applied to people too. By taking a few non-reproductive cells from adult
mammals, identical replicas can be created without damage (or even inconvenience) to the
donors." Nature 402, 743 - 746 (1999) © Macmillan Publishers Ltd.
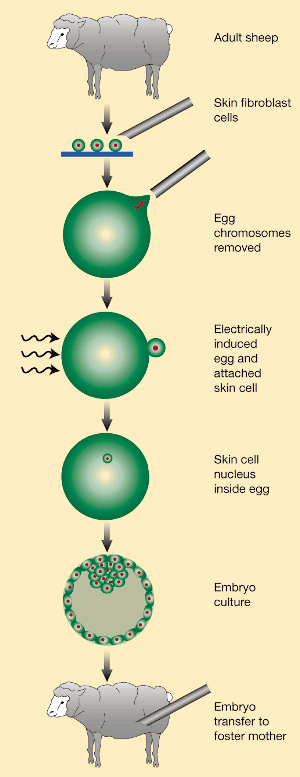
The DNA removed from an adult cell for a cloning experiment must be
"reprogrammed" to restore its ability to be totipotent from its terminally
differentiated state in the adult cell. The microenvironment of the enucleated egg
must somehow stimulate this reprogramming. However, it must be a difficult thing to
do since success is limited. "The pattern of gene expression in adult cells is very
different from that in embryonic cells. In amphibians, for example, a number of genes
expressed in embryos five hours after fertilization are not expressed in differentiated
(specialized) larval or adult cells16. Conversely, some genes are expressed in adult cells
but not in early embryos. When embryos are analysed a few hours after the transfer of
adult cell nuclei, gene expression cannot be distinguished from that in embryos grown from
fertilized eggs. This means that the exchange of cytoplasm around a nucleus, from that of
an adult cell to that of an egg, causes a dramatic switch in gene expression in only a few
hours. A nucleus that was once part of an intestine, skin or muscle
cell is therefore transformed into that of an embryonic cell. "
"New tissues for old"
Damaged or diseased tissues often cannot be repaired by drugs or
other medication, and most organs and tissues regenerate very poorly in mammals. In a few
cases, artificial materials such as replacement joints, or mechanical devices such as
renal dialysis machines, work remarkably well. But ultimately, the most satisfactory
remedy is to transplant organs or tissues from other people.
Transplantation works reasonably well for kidneys, hearts and so on, but there are three
major disadvantages. First, the supply of such organs is extremely limited, depending
largely on donations from accident victims. Second, treatment is very expensive (about
£100,000, or US$160,000, for a replacement heart). Third, recipients need to be given
immunosuppressive drugs to avoid rejection of the transplanted organ owing to the genetic
differences between donor and recipient. Although the use of animal organs has been
considered for
transplantation, the genetic incompatibility is even greater with these, and even animal
organs that have been engineered to contain human immune regulator genes are still targets
for rapid rejection.
An alternative strategy involves stem cells; these are cells that can renew themselves and
also give rise to a variety of differentiated cell types. Mammalian bone marrow, for
example, contains a range of haematopoietic (blood-forming) stem cells. Some of these can
be isolated and encouraged to proliferate using natural signalling molecules such as
members of the interleukin family. These stem cells can then be made to progress to form
more restricted stem cells (which can form a more limited range of cell types) and,
eventually, to form fully differentiated cells such as erythrocytes or granulocytes. This
type of blood stem-cell therapy has been practised for many years in humans, but the
quantity and quality of available stem-cell types is very limited. The prospects also seem
good for stem cells obtained from olfactory placodes. These neural stem cells can be
proliferated in culture, and they have been shown to restore function in the mouse central
nervous system.
It is also possible for stem cells of one type to generate occasional cells of a different
type, although the conditions that achieve this are not known. For example, neural stem
cells can generate haematopoietic stem cells when transplanted to mice that have been
irradiated to eliminate their own blood stem cells. Stem cells from human bone marrow have
been reported to generate functional neural cells. An ideal stem cell is that exemplified
by mouse embryonic stem or germ cells, which are derived experimentally from early mouse
embryos or germline cells respectively. These cells can be proliferated in culture and,
when transplanted to hosts, can differentiate into all types of adult cell. Human cells
with several properties of mouse embryonic stem cells have also been described. However,
all embryonic stem and germ cells are currently obtained by killing normally generated
early embryos, raising ethical concerns for human material.
Despite their advantages, transplantation and stem-cell strategies still suffer from the
problem of immunological incompatibility, a problem that could be avoided if material
derived from a patient's own tissues could be transplanted into them. This is already done
with skin for burns patients, but the amount of material is very limited and, for most
tissues, it is not yet possible to obtain enough stem cells, if they can be obtained at
all. One approach to the problem is to freeze, at birth, samples of cells from the
umbilical cord. This tissue is rich in stem cells which, after proliferation and
differentiation, might be of use later in life.
Therapeutic cloning
Given the problems of rejection, the lack of identified stem cells for most tissues, and the difficulties of using normal human embryos as a source of embryonic stem cells, an altogether different route is to combine therapeutic cloning with the use of differentiation factors. Although not yet practicable, this scheme offers a realistic possibility for the future.
Steps involved in therapeutic cloning.
Our belief, however, is that the greatest eventual benefit of the new technology will be in therapeutic cloning; the use of somatic-cell nuclear transfer to generate replacement tissues or organs. This would avoid the risks of tissue rejection by supplying a person with new tissue of exactly their own genetic type.
Nature 402, 743 - 746 (1999) © Macmillan Publishers Ltd. The future of cloning. J. B. GURDON AND ALAN COLMAN
"Cloning mammals has become something of a cottage industry ever since Dolly first burst on the scene 3 years ago. With the same nuclear transfer technique that spawned Dolly--in which a cultured differentiated somatic cell is fused with a mature egg (oocyte) whose genetic material has been removed (enucleation)--births of live cloned offspring have been reported for sheep, cattle, and goats. However, the animal that is highest on everyone's list to clone but where cloning attempts have met with little success is the pig. Unlikely as it may seem, pigs are physiologically very close to humans and so there has been intense interest in using cloned pigs as organ donors for transplantation to humans (xenotransplantation). Finally, success has been achieved by Onishi et al. who report the birth of a live cloned piglet called Xena, and Polejaeva et al., who recently delivered five healthy cloned piglets .The saga of eggs and bacon. When a donor somatic cell nucleus is transferred by cell fusion or microinjection to an enucleated oocyte, factors in the donor cell cytoplasm that are specific for that donor cell are also transferred to the oocyte.
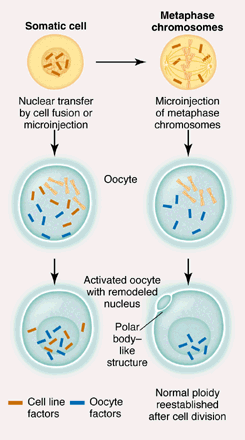
(Left in figure above) These factors along with oocyte-specific proteins become incorporated into the remodeled donor nucleus when it forms after oocyte electroactivation. If too many donor cell-specific factors are transferred to the oocyte, oocyte-specific factors become "diluted" making reprogramming of the donor nucleus less likely. (Right in figure above) If donor metaphase chromosomes instead of a complete donor nucleus were to be microinjected, the resulting remodeled nucleus in the activated oocyte would be assembled with only oocyte-specific factors, and reprogramming of the donor chromatin would become much more likely." (The phase of mitosis, or cell division, when the chromosomes align along the center of the cell. Because metaphase chromosomes are highly condensed, scientists use these chromosomes for gene mapping and identifying chromosomal aberrations.) Volume 289, Number 5486, Issue of 15 Sep 2000, pp. 1886-1887. Copyright © 2000 by The American Association for the Advancement of Science
"When she arrived in Oregon in 1997, prospects looked fairly
bright. Oregon's Don Wolf had just succeeded in using nuclear transfer to produce two
monkeys, Neti (Nuclear Embryo Transfer Individual) and Ditto, a year earlier. As Granada
had done with cows in the 1980s, Wolf had used nuclei from embryonic cells--far easier to
work with than adult cells. But 300 attempts and no pregnancies later, the picture
"is not as rosy," Dominko says. Cloning primates "is not just around the
corner." Neither she, working with Oregon's Gerald Schatten, nor Wolf's team working
one floor below, has been able to replicate Wolf's early success.
Once Dominko realized what she was up against, she tried to determine whether the problem
was with nuclear transfer or the in vitro procedures. She attempted "mock"
nuclear transfers, in which she and her colleagues went through the cloning procedure but
didn't actually replace the egg's own DNA. Instead, they fertilized the egg in vitro after
poking and prodding it the way they would have for a true nuclear transfer
experiment, and then placed it in a female for gestation.

Lonesome twosome. Cloned from embryonic cells, Neti and Ditto are still the only cloned
primates, despite years of effort by several groups.
Those attempts didn't work well, suggesting to Dominko that the in vitro procedures were
the problem. She then made "egg-friendly" improvements such as using sperm
extract instead of harsher chemicals to prompt the egg to divide, which helped the
subsequent nuclear transfer experiments. The team produced roughly 45 embryos by nuclear
transfer this way, but none successfully implanted in a surrogate female monkey's womb.
Then Dominko, Schatten, and colleagues began looking at the transferred nucleus itself.
Under a light microscope, the embryo's expanding cluster of cells, known as the
blastocyst, looked just like those seen after successful in vitro fertilization. But a
closer look at these dividing cells, with confocal microscopy, revealed "a whole
gallery of horrors," says Schatten. The new nucleus seemed completely out of sync
with the egg. Even the first cell division had gone awry, as the chromosomes didn't seem
to have copied and separated as they should have. By the eight-cell stage, some cells had
too much DNA, while a few seemed to have none at all.
Dominko and Schatten then took a closer look at the spindle, and in particular at the
centrosomes, which help organize and guide the movement of DNA during cell division,
making sure that each cell gets the right complement of chromosomes. In primates, they
found, the incoming nucleus tends to leave behind one or both of its centrosomes.
"The embryos we were making probably never had a chance," says Dominko. Given
these results, which are still unpublished, Schatten and Dominko have all but given up on
cloning by nuclear transfer until they develop a better understanding of these
abnormalities. Instead, they have turned to embryo splitting, in which the early embryo is
divided in two and gives rise to identical twins, as a means of generating like animals
useful for research (Science, 14 January, p. 317). Dominko and Schatten don't know why
primates are different from cows, but they are convinced that attempts to clone humans
would run up against the same biological roadblocks.
Even Wolf on the floor below is now looking at embryo splitting, but he has not abandoned
nuclear transfer. He's tried nuclear transfer with some 100 embryos, none of which has
established a pregnancy. Indeed, his studies have revealed another source of failures:
Embryos don't develop at the same rate in a lab dish as they do in the womb. It's
important to keep trying, he argues, as clinical studies often require more than the two
identical animals that can be produced by embryo splitting." Volume 288, Number
5472, Issue of 9 Jun 2000, pp. 1722-1727. Copyright © 2000 by The American Association
for the Advancement of Science.
In an experiment that links the hot-button fields of stem cells and
cloning, scientists announced this week that they have cloned mice from embryonic stem
(ES) cells kept in culture. These cells are capable of developing into any type of tissue
but ordinarily can't grow into a complete organism on their own. The technique is still
inefficient, but if researchers can improve it, it could provide an easier way to create
genetically modified mice.
Although sheep, goats, cows, and mice have all been cloned, in most cases the genetic
material came from cells that had not spent much time in culture. In contrast, researchers
report in the current Proceedings of the National Academy of Sciences that they produced
live-born mice from two ES cell lines that had each gone through more than 30 cell
divisions in the lab. Although cells in culture are easy to work with, they often
accumulate mutations. Those mutations can impede embryonic development, so scientists
weren't sure whether long-cultured cell lines would be candidates for cloning.
In the hands of reproductive biologist Teruhiko Wakayama, who created the clones in the
laboratory of Ryuzo Yanagimachi of the University of Hawaii, Honolulu, the cultured cells
seemed to work as well as cells from a living animal. In collaboration with Peter
Mombaerts of The Rockefeller University in New York City, the researchers extracted a
nucleus from an ES cell and transferred it into a mouse egg that lacked a nucleus. Like
all cloning, the procedure was hit or miss, however. In the team's most successful
experiment, it took more than 1000 cloning attempts to obtain 13 mice that survived to
adulthood, notes developmental biologist Davor Solter of the Max Planck Institute for
Immunobiology in Freiburg, Germany. Volume 286, Number 5449, Issue of 24 Dec 1999, p.
2437. Copyright © 1999 by The American Association for the Advancement of Science.
Stem Cells and Cloning Combined:
Recent work with mouse embryonic stem cells has shown new therapeutic possibilities. These cells can be cultured in the lab. New genes can be added to the cell and the effects of the new gene can be assessed by forcing the cells to differentiate in culture (by adding various factors) by by placing the cell back into a blastocyst, an early stage in the development of the embryo. When the organism develops, the function of the gene can be determined.
In a similar way genes in sheep cells (fibroblasts) can by altered by gene addition. ( Also ways have been developed to remove a gene from a cell.) The nuclei from these altered cells can be put into enucleated eggs, in order to clone the altered cells and form sheep with new and desireable traits. Such a procedure would be useful disrupt an gene in pigs (1–3 galactosyl transferase) which would change the surface of pig cells such that they would elicit a much smaller immune response if the cells were used in human therapy. Such genetically modified and cloned pigs cold be the souce of hearts for human transplants (an example of xenotransplants). In another example, sheep without the protein defective in cystic fibrosis (cystic-fibrosis transmembrane-conductance-regulator) could be cloned to produce an animal model to study the disease.
Tissue Engineering
Replacement human tissue may be grown, as they now are for human skin grafts. To grow a whole replacement organ, a scaffold onto which cells can attach and divide must be made. Recenty, a human ear was grown on the back of a mouse. Scaffolding (a synthetic, porous, degradable polyester) was introduced onto which cartilage cells became attached. The goal would be to eventually develop replacement human organs like heart, lung, etc. for use in transplantation.
Here are some interesting web sites describing this technique.