 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
Introduction:
In 1986, Dr. Paul Williams from the University of Wisconsin
published a paper in Science that revolutionized the teaching of plant biology.
In this paper Dr. Williams described a variety of turnip (Brassica
rapa) that his research group had selected to complete its life cycle
quickly. Since these plants could
grow from seed-to-seed in about 35 days meant that several generations per year
could be grown and that these plants would be ideal organisms to use for
classroom and research purposes. In
recognition of their short life cycle the plants were dubbed Rapid cycling Brassica
rapa (acronym RCBr) or simply "Fast Plants�".
To learn more about the history and biology of RCBr visit the visit the Wisconsin
Fast Plants� web site. This site provides a
wealth of information, including the on-line
version of Dr. Williams article and slides/images
of the plants.
Fast Plants� are easy to grow if you restrict the volume of the container in which they grow and provide them with adequate light and sufficient fertilizer. We will grow our Fast Plants� in film can pots and supply them with Osmocote�, which is a pelleted slow-release fertilizer that has a nitrogen � phosphorus � potassium (NPK) content of 14 � 14 � 14. Our growth medium will be a soil-less potting mix that is a 50/50 mix of milled peat moss and vermiculite and it provides a lightweight matrix that has good water-holding ability and provides adequate air to the roots. Jiffy-Mix� is the trade name for one such product. The plants will be grown under banks of cool white fluorescent bulbs with a fluence that is greater than about 150 umol photon m-2 s-1. Fluence, sometimes erroneously called intensity, refers to the amount light an object receives and it is usually expressed as the number of photons (umol) that strike a given surface (m2) in a given period of time (one second).
Fast Plants� must be cross-pollinated to
produce seeds; that is, they do not self-pollinate.
To hand pollinate a flower biologists can use a variety of techniques.
For example, the stamens of one flower can be removed with a forceps and
then dusted directly on the stigma of the flower of a different plant.
We will mimic the natural process of pollination in Brassica
which, for many species, relies on bees. Thus,
we will pollinate our Fast Plants� with a "bee stick" made
from the thorax of a honeybee.
Fast Plants� can be grown in using
inexpensive, recycled materials such as plastic
film cans. An alternate way to grow them is using styrofoam
quads. The directions for both follow.
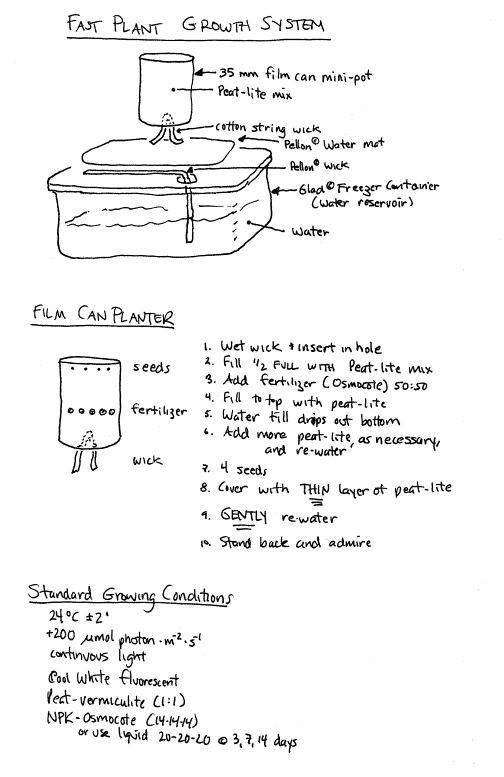
Obtain
several film cans, cotton string wicks, one Pellon� water mat, one Pellon�
wick, and a freezer container. Figure
1 is a diagram of the final set-up.
Punch
a drainage hole in the bottom of each film can with a nail, if this is not
already done.
Wet the wicks by thoroughly soaking them in water and
squeezing out any air.
With
a forceps, grab the string wick in the middle and insert it into the
drainage hole. About half of
the wick should form a loop into the can while the ends should hang from the
film can.
Fill the film can about half full with Jiffy-Mix.
Add 5 fertilizer pellets
Continue to fill the film can with Jiffy-Mix
Holding the film can over the reservoir (freezer
container), wet the mix with water from a squirt bottle until it drips off
the wick. If it doesn't drip
through, you may have to open the drainage hole a little by inserting the
tip of the forceps.
Once moistened, the mix will settle some.
Add more potting mix and rewet until the surface of the mix is at the
top of the film can.
With your thumb, make a shallow depression in the potting
mix. Do not press down too hard
or else you will compact the soil and the plants will not grow well.
Place
four seeds in each film can. Cover with a very thin layer of potting mix.
This layer should be no more than 1 mm thick � or about the
thickness of one seed.
Gently
rewet. Do this carefully or the
seeds may wash to the bottom of the can and not germinate.
The seeds must be near the surface or they won't survive!
Make
label flags with toothpicks and tape. Label
the plants with your name, date, and treatment. Insert the flag into
the soil, not the Styrofoam quad.
Rinse
out the wick and water mat thoroughly.
Place the wick through the hole in the lid of the reservoir and place
the mat on top.
Fill the reservoir with water. With a marking pen and
tape, label the reservoir with your name and other identifying marks.
Place the plants under the lights. The bulbs should at all times be no more than a few
centimeters from the top of the plants.
Make two "bee sticks" for pollinating your
plants. Obtain a dead honeybee.
Carefully remove the head and abdomen.
Place a drop of white glue or Duco cement on the end of a toothpick,
gently skewer it through the thorax and allow to dry. Store the bee
sticks by sticking them in the medium of one of your film cans.
Practice pollinating one of the plants in the lab.
Your instructor will demonstrate the proper technique. To pollinate,
rub the bee stick on the stamens of a flower on one plant.
Then touch the bee stick to stigma of a flower on a different plant.
Then, rub the bee stick in the stamens of this plant and return to
the original plant and rub the bee stick on the stigma.
The seeds should germinate in 1-2 days.
When the seedlings are approximately 3 � 4 days old, thin to two
plants per film can. Use
scissors to cut off the unwanted seedlings at the surface of the growth
medium. Remove seedlings that
look unhealthy, or are excessively tall or short, so that the remaining
seedlings have a uniform appearance.
You will need to pollinate the flowers as they appear,
which should occur when the plants are 12 � 15 days old.
After pollinating 6 flowers on each plant, pinch out the
terminal bud so that no additional flowers develop.
Allow your plants to grow for another 21 days until the
pods and seeds are mature. Remove
the plants from the water reservoir and allow them to dry for a few days.
Collect your data by measuring the pods, counting (and save) the seeds, and/or making any other final observations.
Quads: The basic procedure for growing RCBr in quads is nearly identical to that outlined for film cans. The following will only highlight modifications from the directions above. The advantage of using quads is that they are a little more stable and can handle four plants in a relatively small space. Film cans are nice because they are expendable and inexpensive.
Obtain quads, wicks, water mats and a reservoir. The
reservoir is prepared from a plastic "shoe box" and the water mats
are cut from Pellon interfacing. The wicks are obtained from Carolina
Biological supply, though could be easily made.
Place 3 Osmocote pellets in each cell of the quad.
Plant 2-3 seeds in each cell. Thin seedlings to one per cell.
| | Top | SGS Home | CSB/SJU Home | Biology Dept | Biol 327 Home | Disclaimer | |
Last updated:
01/07/2009 � Copyright by SG
Saupe