TM4. Chelation in Transition Metal Complexes
Each ligand that binds to a transition metal donates a pair of its electrons to the metal, sharing those electrons in a metal-ligand bond. Counting the total number of electrons in the metal's valence shell is fairly easy when you know that each ligand contributes two electrons.
But what if a ligand could contribute more than two electrons? Take a look at the following table. Each of the ligands shown here can bind to a metal twice. The ligand forms two bonds to the metal, donating two pairs of electrons. It might seem obvious, but forming two bonds to the metal means the ligand binds more tightly to the metal. Remember the lego-like nature of transition metal complexes: ligands can come and go, but these ligands are less likely to go; they hold on.
Table TM4.1. Ligands that bind through more than one donor atom.
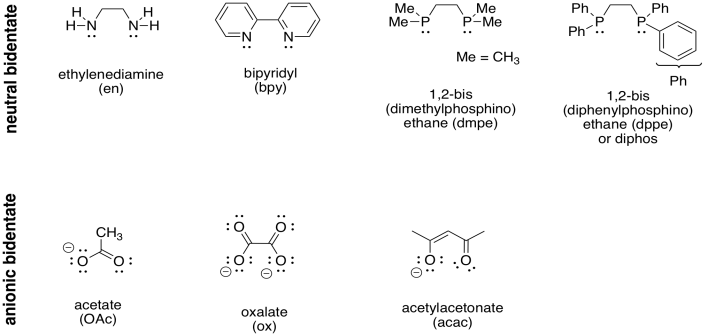
These ligands are called bidentate ligands. That means, literally, that they have two teeth. That doesn't sound like much, but the ligands we have seen previously are described as monodentate; they have only one tooth. Bidentate ligands can bite into the metal, and hold onto it, more strongly than monodentate ones.
Another term used for these kinds of ligands is derived from the Greek chele, for claw. These ligands grab onto the metal like the claw of a lobster or crab; they chelate. The "chelate effect" is the tendency of these ligands to bind firmly to a metal, whereas monodentate ligands might come off more easily.
Now, just because a ligand could be bidentate does not mean that it always binds that way. That's sometimes true with the acetate ligand, for example, because the four-membered ring that forms when it binds through both oxygens is a little too strained. Consequently, there are examples binding through both oxygen atoms, and there are also examples of binding through only one. Sometimes, acetate uses one oxygen to bind to one metal atom and the other oxygen to bind to a second metal atom, forming a bridge.
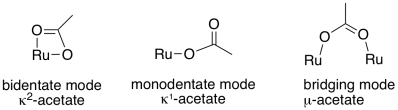
Figure TM4.1. Different binding modes for an acetate ligand.
If a single ligand donates lone pairs through two different atoms, it is often described as binding in κ2 mode, pronounced kappa-two. If the same ligand only uses one of its two possible donor atoms, it is binding in κ1 mode. If the ligand uses one donor atom to bind to one metal atom and the other donor atom to bind to a different metal atom, it is described as binding in μ2 mode, pronounced mew-two. As you might expect, a ligand that binds through three different lone pairs on three different atoms is described as κ3.
Usually, if a ligand is capable of chelation, assume it binds that way. However, there may be cases in which you are asked specifically to draw it binding in one way or another.
Problem TM4.1.
Determine the denticity of each ligand in the following complexes.
a) [Cu(en)2(OH)2] b) [RuCl2(dmpe)(bpy)] c) [Ni(acac)2(OH2)2] d) K2[Cu(ox)2(OH2)2]
e) [FeH2(dmpe)2] f) [RuH(OAc)(PPh3)3] g) [Co(en)2Cl2]BF4 h) [Ru(bpy)2(HOCH2CH3)2](ClO4)2
Problem TM4.2.
Determine the charges (or oxidation states) on the metals in the following complexes:
a) K[Cr(ox)2(OH2)2] b) K[Mn(acac)3] c) [Cr(en)2Cl2]PF6 d) [Co(en)2(OH)Cl]ClO4
e) Na3[Mn(ox)3] f) K3[Cr(ox)3] g) Na[Au(bpy)(CN)2]
Problem TM4.3.
Determine the electron count on the metal in each of the complexes from the previous problem.
See a more in-depth discussion of coordination complexes in a later course.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
Send corrections to cschaller@csbsju.edu Navigation: Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.