Structure in Chemistry
Conformational Analysis
CA11. Other Rings
We have looked at some different six-membered rings, cyclohexanes, and have seen how substituents attached to these rings might influence the shape of the overall molecule. Cyclohexanes are particularly important because of they are quite common in nature. There are other variations of cyclic systems that are worth looking at, including different cyclohexane derivatives and other rings.
Bicyclics and Other Polycyclics
Decalins are examples of fused ring systems. In fused ring systems, two rings share an edge. Decalins contain ten carbons total, but the ten carbons are divided into two rings, each of which is six carbons around.

There are two possible isomers of decalin: cis and trans. The easiest way to distinguish the two isomers is to look for the two hydrogens on the shared edge between the two rings. Those two hydrogens are found on the same face of the cis decalin.
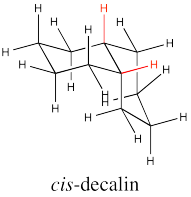
Go to Animation CA11.1. A three-dimensional model of cis-decalin.
The two hydrogens along the junction are seen on the opposite faces in trans decalin.
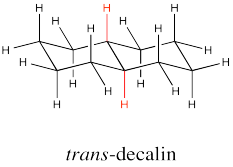
Go to Animation CA11.2. A three-dimensional model of trans-decalin.
Problem CA11.1.
Draw cis-decalin and trans-decalin using a wedge/dash picture.
Problem CA11.2.
Calculate steric strains in cis-decalin and trans-decalin.
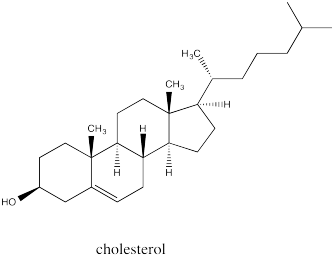
Fused ring systems like decalin are very common. In fact, similar ring systems are found in steroids, which are an important class of lipids. Steroids generally have similar structures that include a series of fused rings as seen in the parent compound, cholesterol.
The similarity is striking between estradiol, a female sex hormone, and testosterone, a male sex hormone.
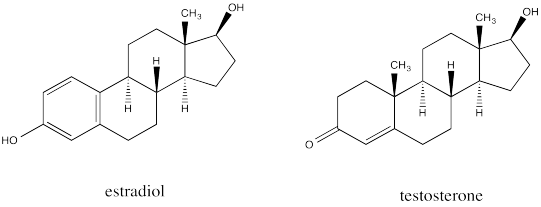
Go to Animation CA11.3. A three-dimensional model of estradiol.
Go to Animation CA11.4. A three-dimensional model of testosterone.
Problem CA11.3.
Consider a steroid structure such as cholesterol.
a) Based on the relationships along the fused edges between the ring, does the structure more closely resemble that of cis-decalin or that of trans-decalin?
b) The overall shape of the structure could probably best be described as (choose one: wide and wavy / compact and curled up / boxy like a cage).
Polycyclic systems do not have to be fused along one edge like decalin. They can be connected in a variety of ways. Norbornane is an example of a bicyclic system in which the two rings actually share more than two carbons.

Norbornane can be used to illustrate the systematic way to describe fused ring systems. The suffix describes the total number of carbons in the ring system. The prefix describes how many rings are fused together. The middle part, in brackets, indicates how many atoms form the "bridges" between the "bridgehead" atoms, where the system splits off into two different rings. One number is given to describe each bridge. In norbornane, there are two 2-carbon bridges and a single 1-carbon bridge.
Problem CA11.4.
Write a systematic name for decalin.
Problem CA11.5.
Provide systematic names for the following structures.
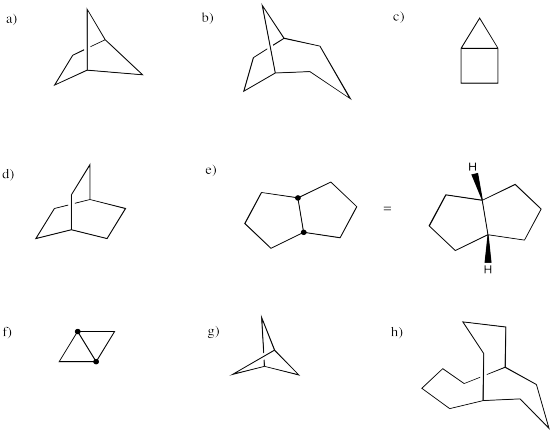
Problem CA11.6.
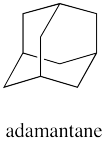
How many different rings can you trace in the structure of adamantane?
Bicyclic systems are common in nature. For example, the tropane alkaloids all contain a bicyclo[3.2.1] ring system. Alkaloids are a group of natural products that contain basic nitrogen atoms. The tropane alkaloids contain this nitrogen atom in the 1-atom bridge. Tropinone is a simple example.
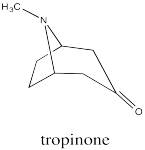
Common examples of natural products containing the tropinone structure include atropine and cocaine. Atropine, derived from deadly nightshade or belladonna, is a heart medication; it is used to increase heart rate if the rate gets too low. Atropine can also be fatal if used incorrectly, causing a wide range of physiological effects; in some dosages, it can even decrease the heart rate. Cocaine, of course, is a narcotic and a controlled substance.
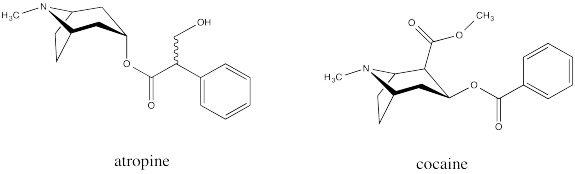
These compounds have a special place in the history of organic and medicinal chemistry. During World War I there was a shortage of atropine, prompting Sir Robert Robinson to develop an efficient synthesis of tropinone as an inexpensive source of synthetic atropine.
Larger Rings
Larger rings of carbon atoms are possible, although they are not as common as five- and six-membered rings. Ring formation is thought to be easiest from a six-carbon chain, because the two ends of the chain can easily wrap around to connect together yet they are not so distant that such an encounter becomes unlikely.
Intermediate-sized rings, such as cyclodecane, become distorted to avoid steric interactions. Internal steric interactions arean added complication in the formation of some rings.
Problem CA11.7.
Show the steric interactions within cyclodecane.
At some point, in very large rings, steric problems are not as severe. The hydrogens on the inside of the ring may be far enough away from each other to avoid interactions. Cyclooctadecane is an example of such a ring. Although interior 6-atom interactions are possible, the ring is flexible enough to twist in such a way as to avoid these problems.
Problem CA11.8.
Look at the model for progesterone and make a line drawing of it.
Go to Animation CA11.5. A three-dimensional model of progesterone.
Problem CA11.9.
Look at the model for androstanediol and make a line drawing of it.
Go to Animation CA11.6. A three-dimensional model of androstanediol.
Still pictures of models obtained using Spartan 14 from Wavefunction, Inc., Irvine, California.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: