Structure in Chemistry
Conformational Analysis
CA15. Solutions to selected problems
With contributions from Dr. Edward McIntee, CSB/SJU
Problem CA3.1.

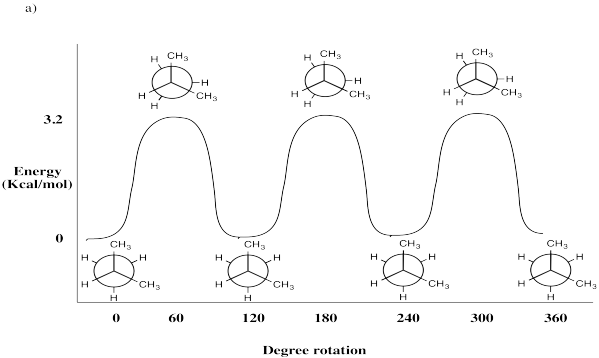
Problem CA4.1.
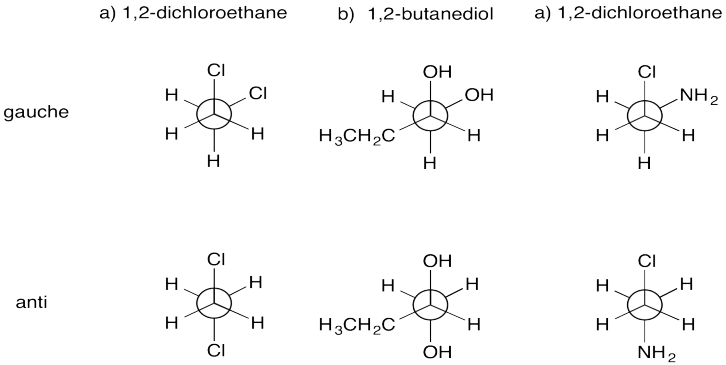
Problem CA4.2.
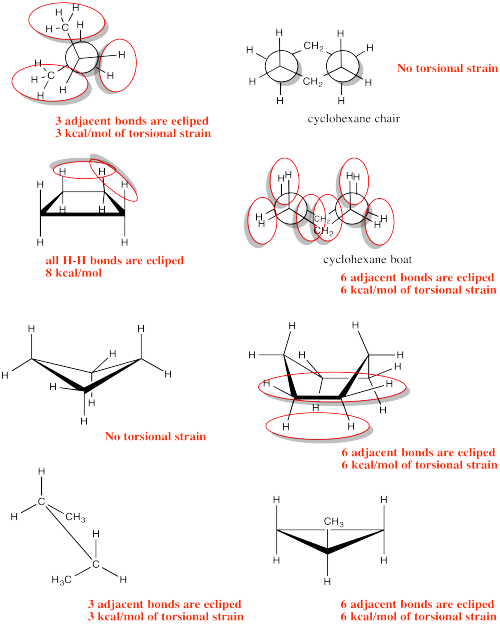
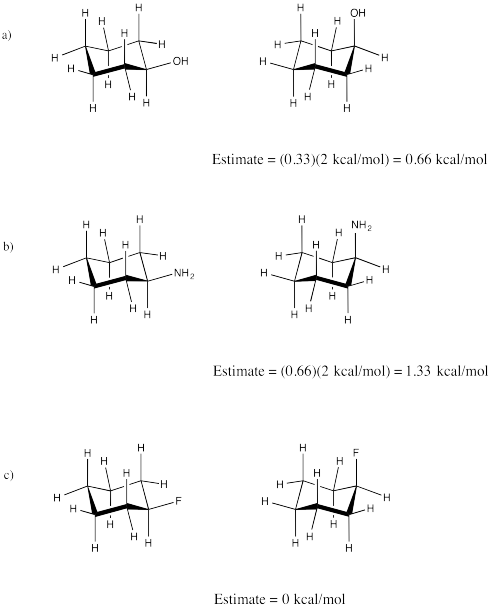
Problem CA4.3.a.
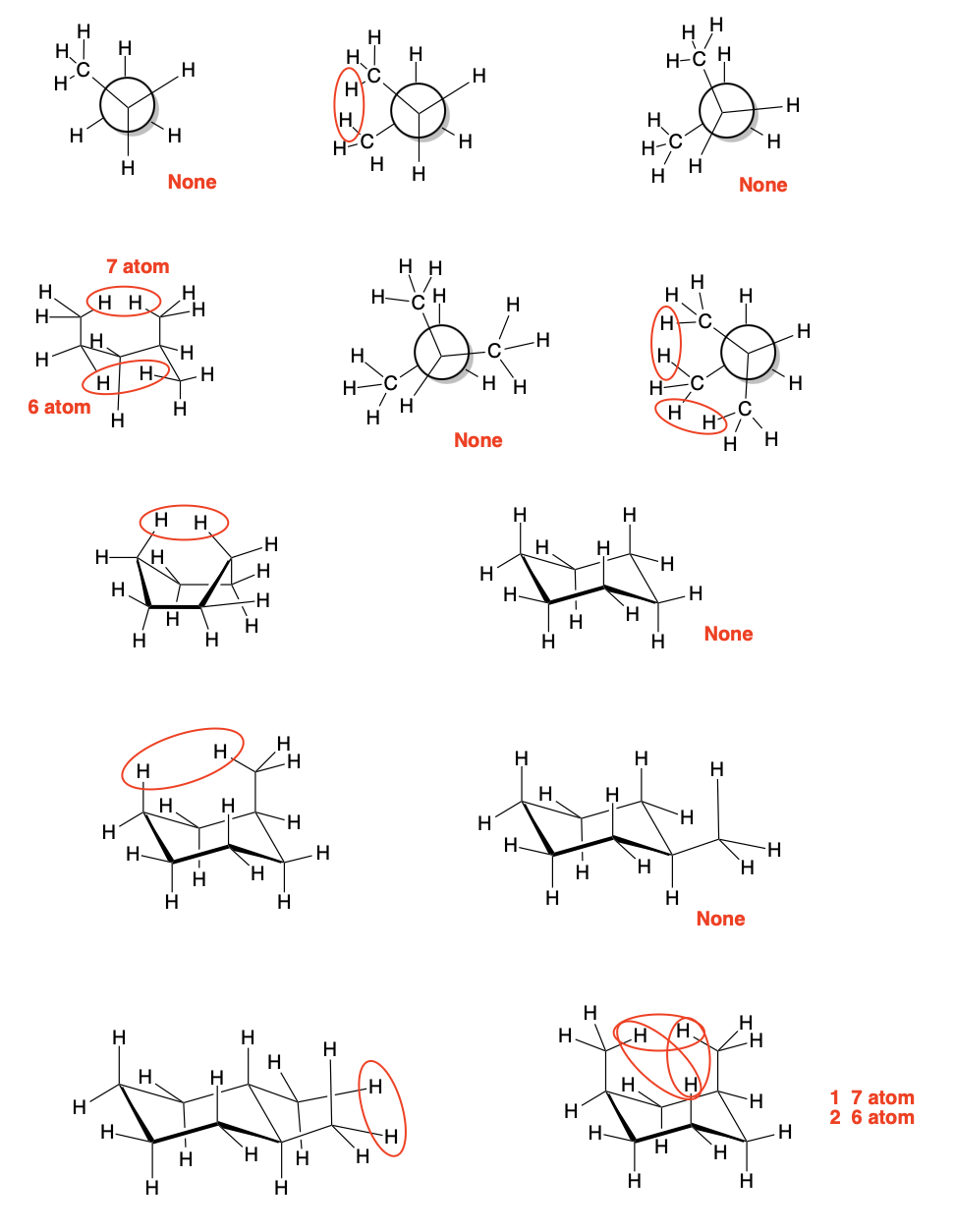
Problem CA4.3.b.
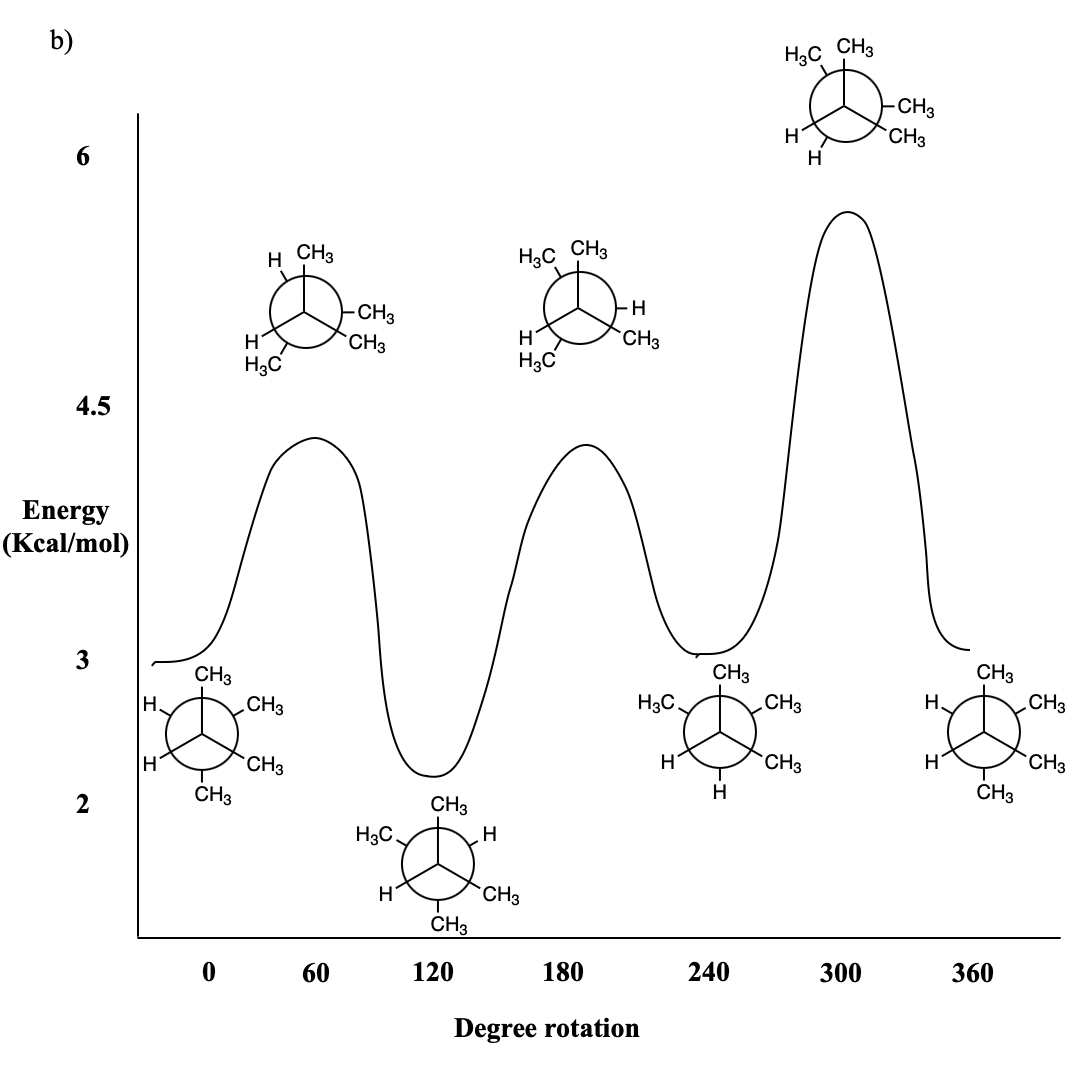
Problem CA4.3.c.
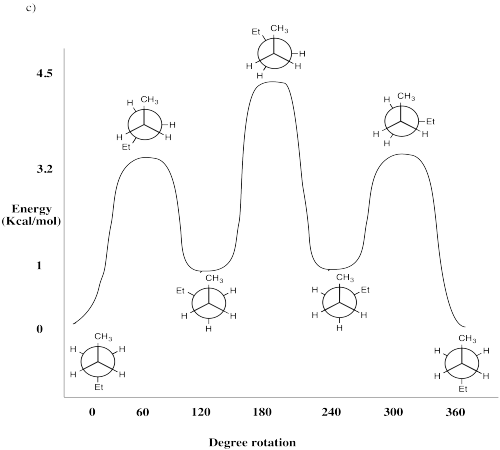
Problem CA8.1.
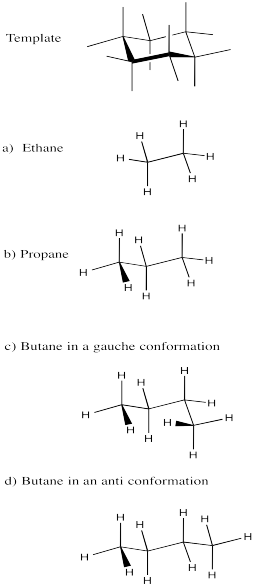
Problem CA9.1.
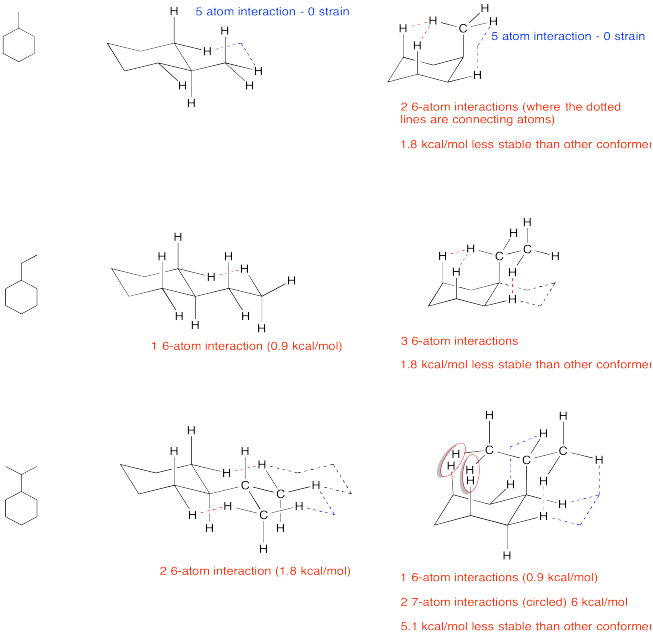
Problem CA9.2.
The statistical factor takes into account the number of staggered conformations of the axial group that would lead to a 1,3-diaxial interaction.
With a CH3, every such conformer would show a 1,3-diaxial interaction. With an OH, only 1/3 of them would. The steric strain for an axial OH is thus 1/3 (1 kcal/mol) = 0.33 kcal/mol.
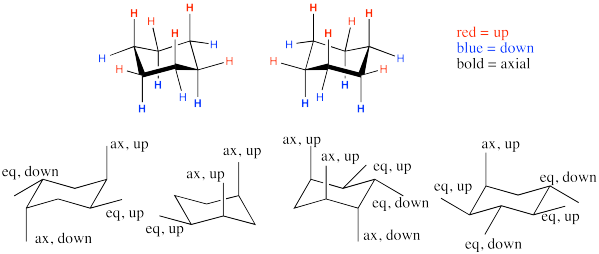
Problem CA9.3.
Problem CA10.0.
The two conformers are equal in energy. There would be a 1:1 mixture of these two forms.

Problem CA10.1.
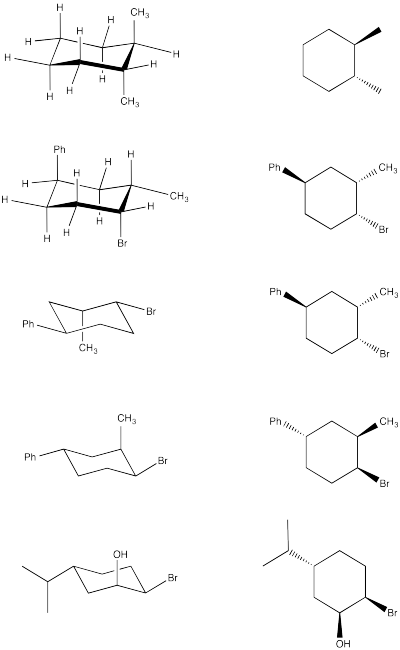
Problem CA10.2.
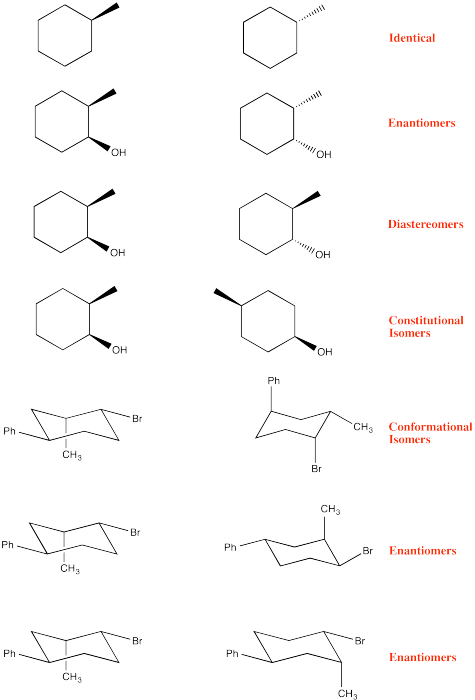
Problem CA10.3.
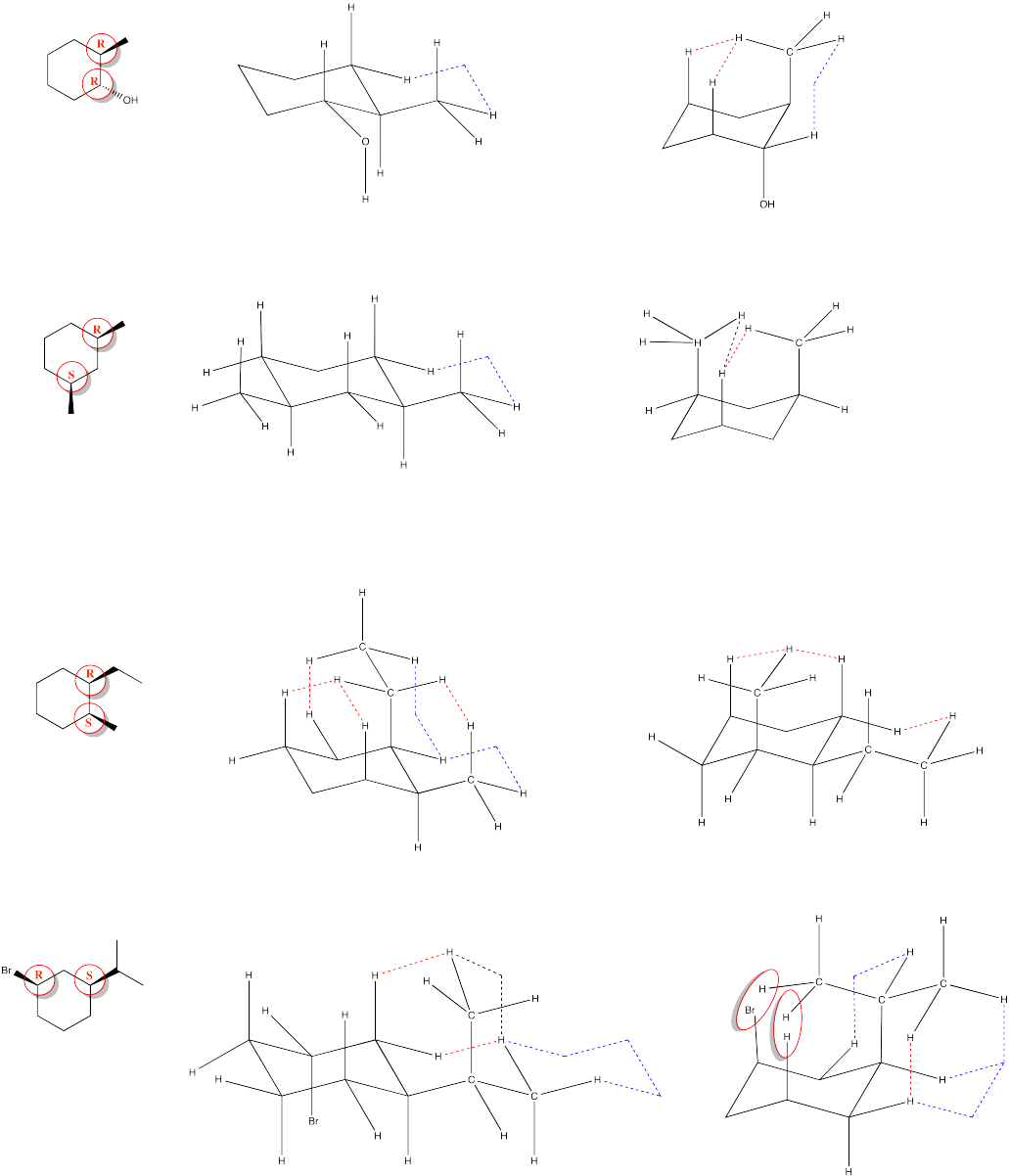
Problem CA10.4.
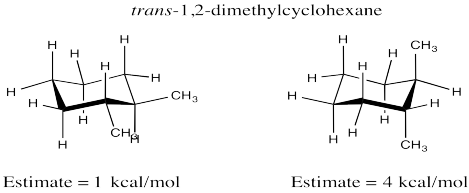
Problem CA10.5.
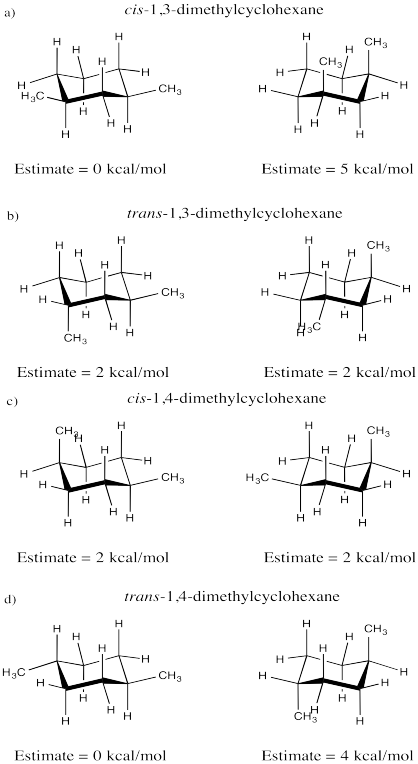
Problem CA11.1.
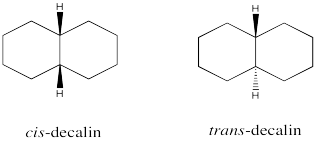
Problem CA11.2.
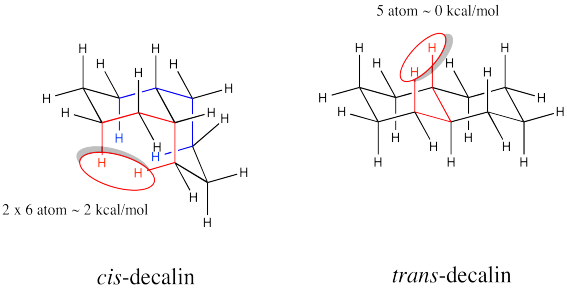
Problem CA11.3.
a) The substituents are always trans along the junctions between each pair of rings. The steroids resemble a series of trans-decalin structures.
b) The overall structure would be more wide and wavy like a trans-decalin, rather than curled or boxy like a cis-decalin.
Problem CA11.4.
Bicyclo[2.2.0]decane
Problem CA11.5.
a) Bicyclo[2.1.1]hexane b) Bicyclo[3.2.1]octane c) Bicyclo[2.1.0]pentane (more commonly called "housane")
d) Bicyclo[2.2.2]octane e) cis-Bicyclo[3.3.0]octane
f) cis-Bicyclo[1.1.0]butane g) Bicyclo[1.1.1]pentane h) Bicyclo[4.3.3]dodecane
Problem CA11.6.
Although we could sketch out many rings using adamantane, just three rings are needed to include all the carbon atoms in the structure. Thus, adamantane is considered a tricyclic system. The systematic nomenclature of tricyclic systems gets a little more complicated, so we won't worry about that.
Problem CA11.7.
If cyclodecane adopted a regular diamond lattice conformation, there would be a whopping 8 atom interaction in the middle of the ring. That interaction isn't even included in our basis set. It would cost at least 6-7 kcal/mol. As a result, the cyclodecane adopts a twisted structure to avoid this interaction.
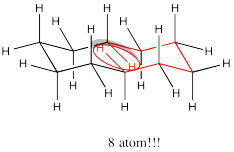
Problem CA11.8.
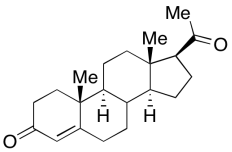
Problem CA11.9.
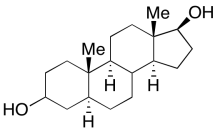
Problem CA12.1.
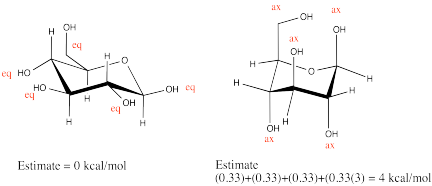
Problem CA12.2.
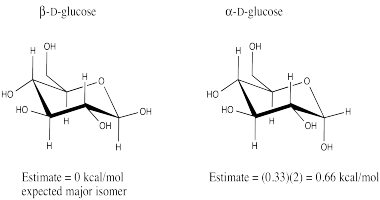
Problem CA12.3.
a) The β-D-glucose isomer should be the more stable isomer. The β-D-glucose
isomer places the C1 hydroxyl group in the equatorial position.
b) The
β-D-glucose isomer should be the more abundant isomer.
c) This is due to
something called the anomeric effect. In solvents of modest polarity, such as
dicholoromethane, the α-D-glucose isomer is not as polar as the β-D-glucose
isomer. In the α-D-glucose isomer the dipoles of the ring oxygen and the C1
hydroxyl group opposing each other (therefore the overall effect is the molecule
is less polar). In addition, the α-D-glucose isomer is stabilized by hyper
conjugation of the ring oxygen and C1. For more information see
http://en.wikipedia.org/wiki/Anomeric_effect
d) A more polar environment
would promote having more of the β-D-glucose isomer around. In the β-D-glucose
isomer, the dipoles of the ring oxygen and the C1 hydroxyl group align each
other (therefore the overall effect is the molecule is more polar).
Problem CA13.1.
Problem CA13.2.
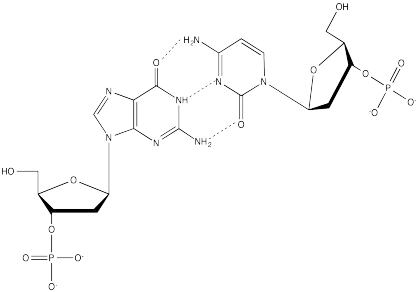
Problem CA13.3.
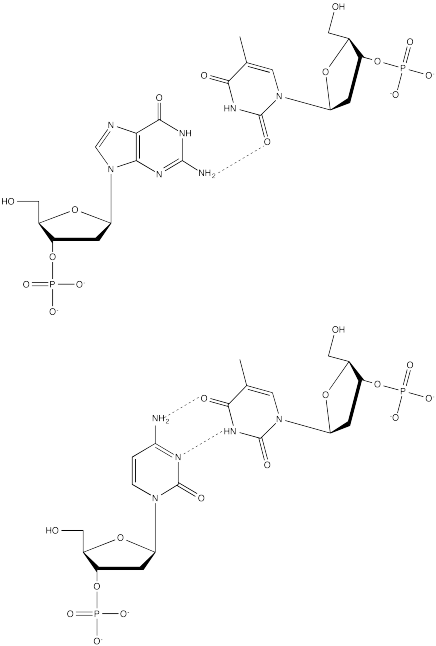
Problem CA13.4.
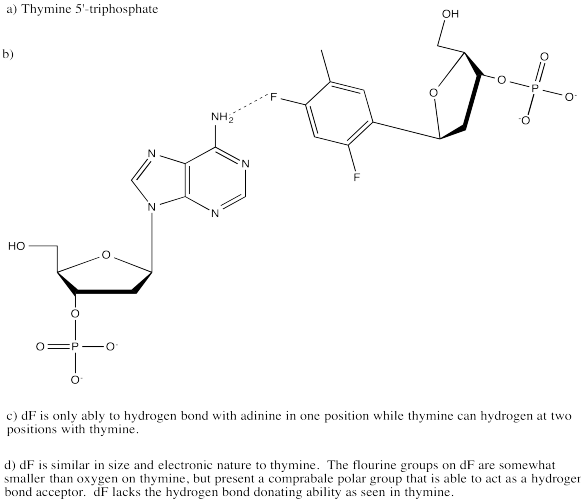
Problem CA13.5.
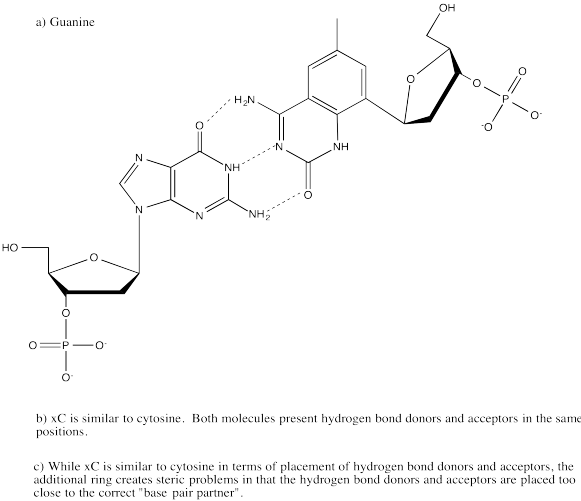
Problem CA14.1.
a)
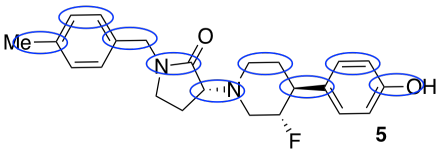
b) about 9 Å
c)
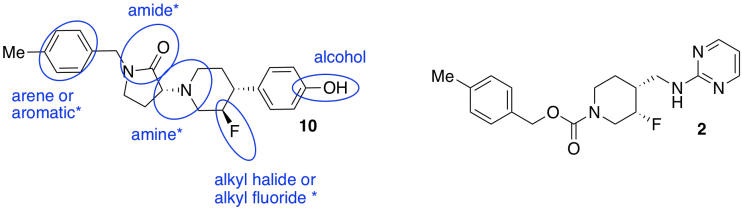
d) Stereochemistry. Because biological receptors are generally proteins containing unique, and chiral, binding sites, they hope to find a stereoisomer that fits the GluN2B receptor, but not the hERG receptor.
e) These compounds are diastereomers of each other.
f)
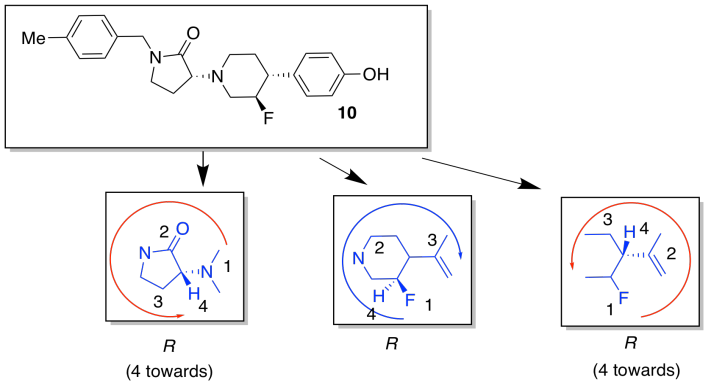
g)
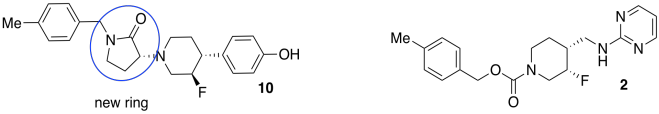
h)
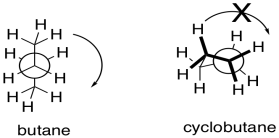
The ring introduces conformational rigidity by preventing the bond from undergoing complete rotation; it is tied back by the connection on the other side of the ring.
i) They were trying to decrease the number of possible conformations, or shapes, that the compound could adopt. That way, it would be less likely to bind in both the GluN2B receptor and the hERG receptor.
j) The substituents around the flexible six-membered ring could adopt a number of conformations. We will choose ways that minimize additional steric interactions so that we can focus on the most basic interactions.
Note that amines do not usually have fixed stereochemistry; the fourth substituent on an amine nitrogen is a lone pair, which does not occupy a fixed position in space. Unlike a carbon, the nitrogen can readily switch from one stereochemistry to another.
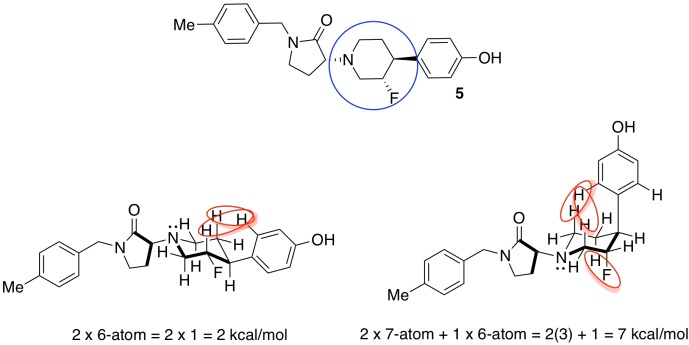
The one on the left, with more equatorial substituents and thus fewer steric interactions, is the more stable one.
Problem CA14.2.
a)
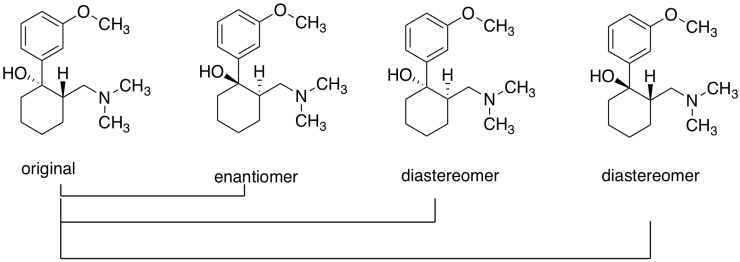
b) The original and its enantiomer; the first two above, from the left.
c)

d) The one on the left, with both groups equatorial and less steric strain, is more stable.
e) If the one on the left is active, a lower dose can be used because it will spend more time in the active form and be able to bond more receptors. If the less stable one, on the right, is the active form, a higher dose will be needed because only a small fraction of the molecules will be ready to bind the receptor at any given time.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: