Structure & Reactivity in Chemistry
Structure-Property Relationships
SP6. Hydrogen Bonding
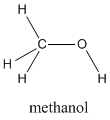
Methanol, CH4O (or CH3OH, a CH3 group attached to an OH), is another example of a molecule with a similar molecular weight to ethane. Like methanal, methanol freezes somewhere around-90 oC, but it does not become a gas until it is heated to 65 oC. Methanol is certainly similar to methanal in some ways. It contains oxygen and is very polar. The huge difference in their boiling points is due to the very strong hydrogen bonds in methanol.

Figure SP6.1. Methanol.
Hydrogen bonding occurs when there is a significant amount of positive charge building up on a hydrogen atom. That happens because the hydrogen is attached to an atom that is much more electronegative than the hydrogen. As a result of that positive charge, a lone pair on another molecule strongly interacts with the hydrogen.
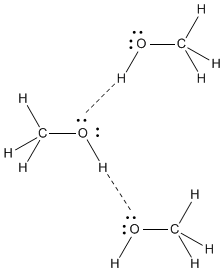
Figure SP6.2. Hydrogen bonding between methanol molecules.
Hydrogen bonds occur only when a hydrogen is attached to one of the few most electronegative elements in the periodic table: fluorine, oxygen or nitrogen. It is these strong hydrogen bonds that are responsible for the relatively high boiling point of methanol; there is so much positive charge on the hydrogen of the OH group that it can essentially form a real bond with the lone pair on another methanol molecule.
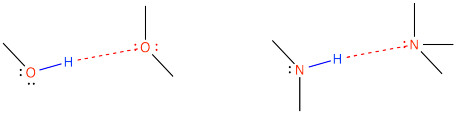
Figure SP6.3. Alcohols as well as secondary and tertiary amines are commonly involved in hydrogen bonds.
Water is an even better example of a hydrogen bonding compound; in fact, water molecules are so tightly bound to each other that this very small molecule freezes at 0 oC and does not boil until 100 oC (way, way hotter than an August day in Las Vegas). Water molecules are much less able to move around freely than are ethane molecules, even though ethane molecules weigh almost twice as much.
For practical purposes, hydrogen bonding usually involves compounds that contain N-H and O-H bonds. That's because oxygen and nitrogen are the second- and third-most electronegative elements in the periodic table, respectively. There are many, many examples of compounds like that. It also occurs in HF, of course, because fluorine is usually considered the most electronegative element, but there is really only one example of a compound with that bond, so you are much less likely to have to worry about that example. Slightly weaker hydrogen bonds have also been shown to occur involving other species, such as S-H bonds, but the most significant ones involve N-H and O-H bonds.
Problem SP6.1.
Which of the following molecules are capable of strong hydrogen bonding?
a) ethanal, CH3CHO b) ethylamine, CH3CH2NH2 c) acetic or ethanoic acid, CH3CO2H d) dimethyl peroxide, CH3OOCH3
Problem SP6.2.
Predict the relative order of melting points in the following amines:
hexylamine, CH3(CH2)4CH2NH2; triethylamine, (CH3CH2)3N; dipropylamine, (CH3CH2CH2)2NH
Problem SP6.3.
Identify the strongest IMF in each case.
Biological Molecules and IMFs
Because compounds containing oxygen and nitrogen are so common in biology, hydrogen bonding is a fairly common feature in organic and biological chemistry.
Carbohydrates are one of the major classes of biomolecules. Carbohydrates are compounds that contain lots of OH groups, so they have lots of potential to form hydrogen bonds.
Proteins and peptides contain amide groups. Amides contain N-H bonds; hydrogen bonds involving amides are crucial influences in the structure of proteins.
Large biological molecules like proteins consists of 1000s of atoms, which interact through "intramolecular" ion-ion, London dispersion, dipole-dipole, and hydrogen bonding to create a unique 3D structure. Other molecules, both small and large, can bind to biological macromolecule using the same intermolecular interactions. An example of a protein, bovine low molecular weight protein tyrosyl phosphatase, is shown below.
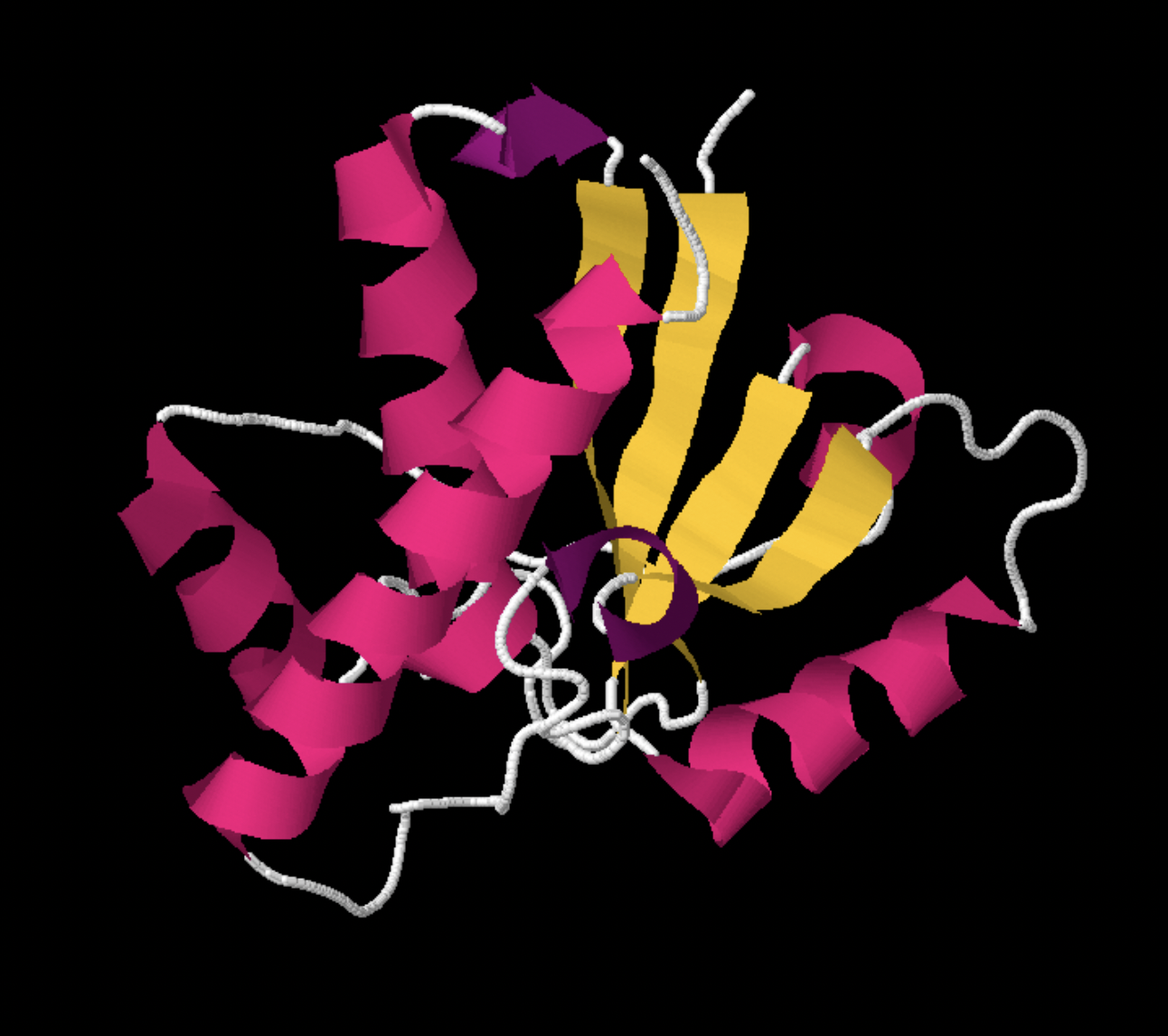
Figure SP6.4. A "ribbon" or cartoon drawing of a protein, bovine low molecular weight protein tyrosyl phosphatase.
Go to Animation IM14.1. A three-dimensional model of bovine low molecular weight protein tyrosyl phosphatase, binding to inorganic phosphate.
DNA may be the most well-known example of a compound that displays hydrogen bonding. In DNA, complementary "base pairs" hydrogen bond to each other to held two strands of DNA together.
Weak, "Secondary" Hydrogen Bonds
Hydrogen bonding is really a special case of dipole forces. It occurs under very particular circumstances: in the presence of O-H or N-H bonds, which provide both a very positive hydrogen and a lone pair, the two features necessary for a hydrogen bonding interaction.
In general, we think of a hydrogen bond as the electrostatic interaction between an electron pair on one atom and a hydrogen atom that has built up a partial positive charge. There are some examples where that might be happening to a small extent, but that we would rather keep separate from the general idea of a hydrogen bond. For example, a lone pair on an oxygen atom in methanal might be attracted to a hydrogen on another methanal, which would be at the more positive end of the molecule. There is evidence from x-ray crystallography that such weak "hydrogen bonds" occur in the solid state in molecules containing aldehyde groups, similar to the one in methanal.
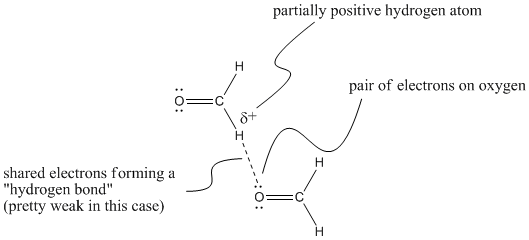
Figure SP6.5. Very weak hydrogen bonds between methanal molecules.
However, these kinds of interactions are really very weak. They shouldn't be thought of in the same category as regular hydrogen bonds. For that reason, we will give them a separate term, "secondary" hydrogen bond. This type of interaction is fairly esoteric and you are not that likely to see it. We're looking at it here really to underscore the idea that there is something very special about the hydrogen bonds resulting from compounds that contain O-H and N-H bonds.
Later, you will see that whenever there is a case in which there is an opportunity for regular hydrogen bonding, the properties of the molecule are dramatically affected. That isn't true otherwise. Secondary hydrogen bonds may be responsible for some very subtle effects in the solid state, when molecules are already at pretty low energy and are not moving around much. However, they hardly change the properties of a molecule beyond what we would expect based on simple dipole-dipole interactions.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: