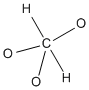
Figure IM4.1. A carbon atom with a problem: too many valence electrons.
Structure & Reactivity in Chemistry
Introduction to Molecules
IM4. Lewis structures and polyatomic molecules
Connectivity
Thinking about how atoms and electrons are arranged in a molecule gets harder when there are more than two atoms. Often you need to decide how the atoms are arranged before writing the Lewis structure.
Let's draw the structure for carbonic acid, H2CO3. First we need to know which atoms are connected together. That will tell us which atoms are sharing electrons.
We could put one atom in the middle of the structure and arrange all the others around it. That may work sometimes, but often it gives the central atom too many bonds. A second-row atom can't have more than eight electrons in its valence shell. As a result, a carbon can't have more than four bonds, because there is no room for more electrons.
Figure IM4.1. A carbon atom with a problem: too many valence electrons.
We could try to attach all the atoms in a string. Long strings of atoms like this are not very common. There may be several reasons why this sort of arrangement isn't very stable. First, when we consider formal charge a little later, you will see that placing all the atoms in a row may cause unnecessary charge separation. Second, some atoms such as oxygen are not very stable when bonded to other atoms of the same kind. Repulsion between lone pairs may contribute to this instability. Because carbon often has no lone pairs, it is an exception and it can bond to itself.
Figure IM4.2. A long string of atoms may still have problems when you start adding in the rest of the electrons.
Note that when we try to arrange the atoms in either way, we would probably avoid putting a hydrogen in the middle. That's because hydrogen's octet is just two electrons, so it can usually only make one bond. Knowing the number of bonds that other atoms usually form can also be helpful.
Figure IM4.3. Hydrogen atoms with problems: usually, atoms with lower numbers of valence electrons must be nearer the edge of the molecule.
Valence
Valence is the number of bonds an element usually forms - for example, the valence of carbon is four, nitrogen is three, oxygen is two, fluorine and hydrogen are one. Valence usually corresponds to the number of electrons needed to form an octet. However, there are exceptions: boron would need 5 electrons to form an octet, but since it only has three electrons to share, it can only form three covalent bonds.
Table IM4.1. Typical valences (number of bonds formed) for several second-row atoms.
| atom | B | C | N | O | F |
| valence | 3 | 4 | 3 | 2 | 1 |
Keeping valence in mind can help Lewis structures go more easily. In the case of carbonic acid, carbon might go in the middle, since it must form the most bonds to obtain an octet. Also, having two of the oxygens connected to hydrogens as well as to carbon helps them to attain their normal valence as well.
Figure IM4.4. Knowledge of valence suggests the structure on the right is more likely.
Once the connectivity has been filled in, we have a skeletal structure of the compound. Now we just need to fill in the extra electrons. Six from each oxygen is 18; four from carbon makes 22; one from each hydrogen makes 24 total electrons.
Figure IM4.5. Adding in the remaining 14 electrons leads to this structure.
Coming up with a Lewis structure requires a number of steps, but it usually follows a familiar pattern.
Figure IM4.6. A set of instructions for constructing a Lewis structure.
Problem IM4.1.
Use the idea of valence to construct Lewis structures of the following compounds:
a) hydroxylamine, H3NO b) ethene, C2H4 c) ethane, C2H6
Problem IM4.2.
Elements in the third row of the periodic table often behave similarly to elements in the second row. Use the idea of valence to construct Lewis structures of the following compounds:
a) PH3 b) CS2 c) SiH4 d) PCl3
Sometimes there will be more than one "correct" way to draw a Lewis structure for a given set of atoms. As an example, there are two different structures in nature with the formula C2H6O. These compounds, methyl ether and ethanol, are called isomers, meaning they are formed from the same (iso) things (mer).
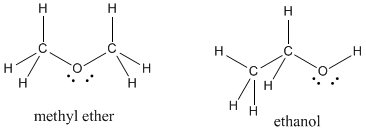
Figure IM4.7. A pair of isomers.
To help distinguish between isomers, formulae are sometimes written with structural abbreviations. Instead of writing C2H6O for both these compounds, we would write CH3OCH3 for methyl ether, suggesting the first carbon is connected to three hydrogens and an oxygen, the oxygen is connected to the second carbon, and the second carbon is connected to another three hydrogens.
In a similar way, ethanol would be abbreviated as CH3CH2OH.
Problem IM4.3.
Draw Lewis or Kekule structures for the following compounds:
a) propanol, CH3CH2CH2OH. b) acetic acid (or ethanoic acid), CH3CO2H
c) methylamine, CH3NH2 d) butanal, CH3CH2CH2CHO
Problem IM4.4.
Draw at least two isomers for each of the following formulae:
a) C3H6 b) C4H10 c) C4H8 d) C3H6O
Sometimes, the decision about connectivity involves additional factors, other than valence. Here is a more detailed set of instructions:
Steps for Guessing Connectivity
If the connectivity is not provided and you need to draw a Lewis Structure,
A. The first atom listed in the formula is often in the middle of the structure.
B. Most simple structures, especially for inorganic species, have an atom in the middle with several atoms connected to and branching out from this central one (as opposed to a linear chain of atoms). Thus, choose the middle atom to be:
1. The atom in the lowest row of the periodic table is the biggest, so it can fit the most other atoms around it.
2. The atom more in the center of the periodic table (group 4, then 3 or 5, etc) has highest valence.
3. Combining these two ideas, we sometimes say he least electronegative atom is often in the middle.
C. Many simple structures are symmetric. This is not a law of nature but a simple observation!
X-M-X often favored over X-X-M
D. Oxyacids often have H�s on O�s; for example, HClO4 has the connectivity H-O-ClO3. The chlorine is attached to four different oxygen atoms, but one oxygen atom is also attached to a hydrogen.
E. Avoid three and four membered rings--they are not common, especially among inorganic species.
F. Avoid bonds between electronegative atoms (F-F, O-O, O-F, etc) . Such bonds are weak and tend to make molecules unstable, if not non-existent. Obviously the situation cannot be avoided in molecules like O2, OF2, F2, etc.
Problem IM4.5.
Provide structures for the following compounds. Remember to think about common valences.
a) NF3 b) N2H4 c) BF3
d) SiF4 e) Ge2H6 f) SnCl4
g) ClNO h) AlBr3 i) HCN
j) S8 k) P4 l) P4O6
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure &
Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Structure & Reactivity Web Materials