Structure & Reactivity
Infrared Spectroscopy
IR1. Introduction
This chapter will focus on infrared (IR) spectroscopy. The wavelengths found in infrared radiation are a little longer than those found in visible light. IR spectroscopy is useful for finding out what kinds of bonds are present in a molecule, and knowing what kinds of bonds are present is a good start towards knowing what the structure could be.
What does an IR spectrum look like?
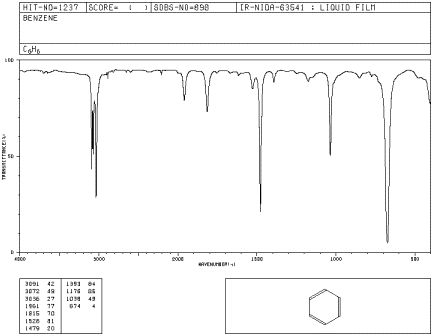
A spectrum is a graph in which the amount of light absorbed is plotted on the y-axis and frequency is plotted on the x-axis. An example is shown below. You can run your finger along the graph and see whether any light of a particular frequency is absorbed; if so, you will see a "peak" at that frequency. If not, you will see "the baseline" at that frequency.

Figure IR1.1. IR spectrum of benzene.1 The x-axis labels are, from left to right, 4000, 3000, 2000, 1500, 1000 and 500 cm-1.
In IR spectra (spectra = plural of spectrum):
As you run your finger from left to right across an IR spectrum, you can see whether or not light is absorbed at particular frequencies. When the curve dips down, less light is transmitted. That means light is absorbed. The dip in the graph is called a peak. Different bonds absorb different frequencies of light, so the peaks tell you what kinds of bonds are present.
Ref. 1. Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: