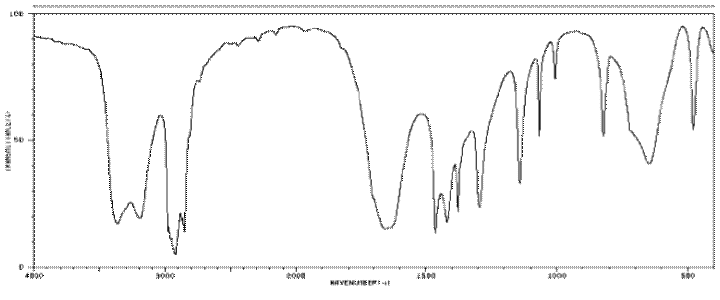
IR16. Sketch an approximate IR spectrum for each of the following compounds:
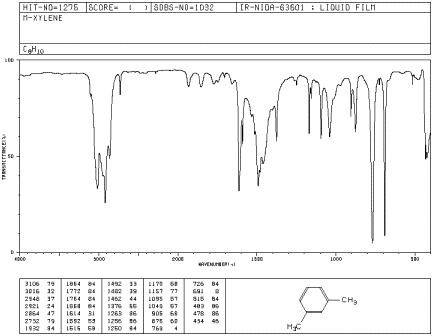
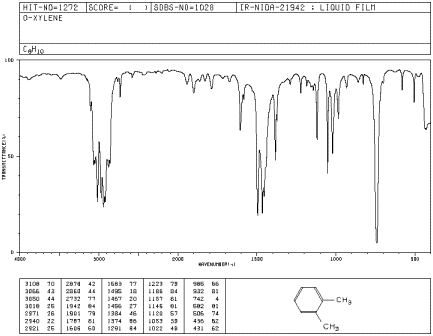
IR17. The oop bends are sometimes useful in distinguishing substitution pattrens around a benzene ring. Using the spectra of o-, m-, and p-xylene, formulate some guidelines about what the oop bends look like when substituents are one, two or three carbons away on a benzene ring.
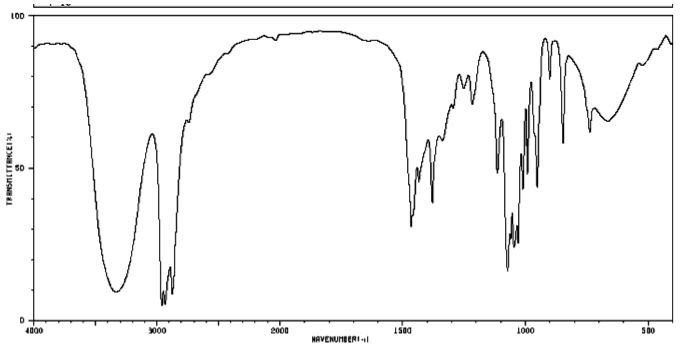
Figure IR18. IR spectrum of m-xylene.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
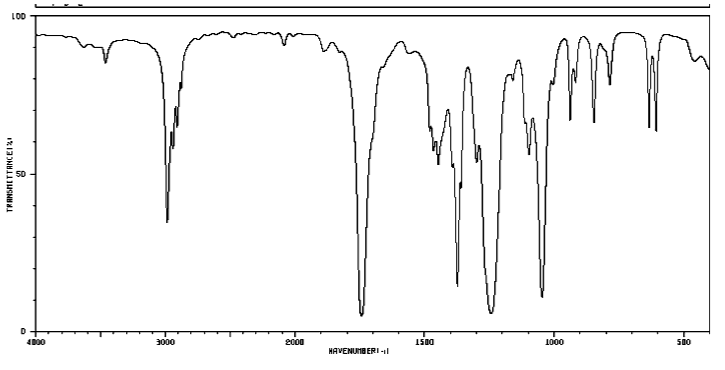
Figure IR19. IR spectrum of o-xylene.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
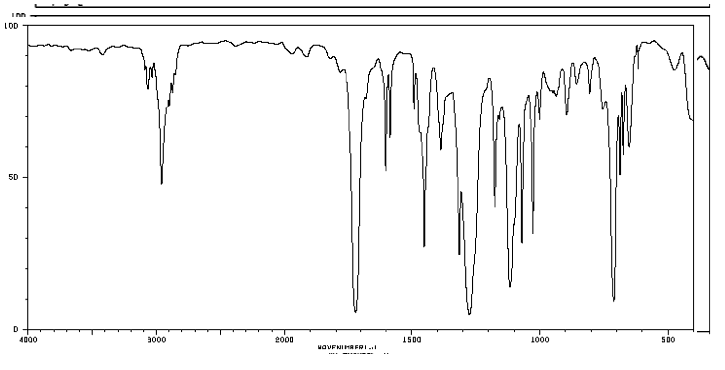
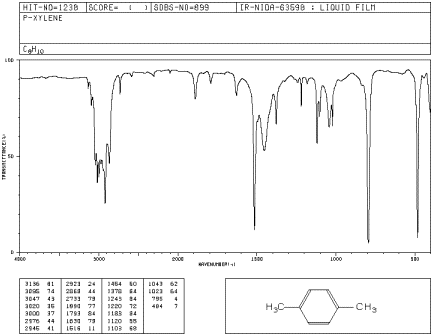
Figure IR20. IR spectrum of p-xylene.
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
IR18. Sketch what the following IR spectra might look like:
a) a spectrum of 2-butanone, CH3COCH2CH3, contaminated with a roughly equal amount of 2-butanol, CH3CHOHCH2CH3.
b) a spectrum of 2-butanone, CH3COCH2CH3, dissolved in tert-butyl methyl ether, (CH3)3COCH3, to make a 1% solution.
These materials are protected by copyright 2008, Chris P. Schaller
Navigation:
Back to Structure & Reactivity
Back to Organic Chemistry Web Materials