IR4. Carbon-Carbon Multiple Bonds
Unsaturated hydrocarbons contain only carbon and hydrogen, but also have some multiple bonds between carbons. One type of unsaturated hydrocarbon is an olefin, also known as an alkene. Alkenes contain double bonds between carbons. One example of an alkene is 1-heptene. It looks similar to hexane, except for the double bond from the first carbon to the second.
![]()
Look at the IR spectrum of 1-heptene. You should see:
- a set of peaks dipping down from the baseline at about 2900 cm-1.
- another set of peaks dipping down from the baseline at about 1500 cm-1.
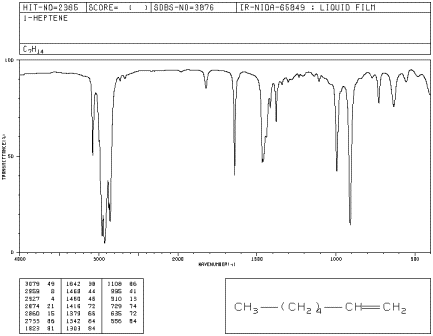
Figure IR4.1. IR spectrum of 1-heptene.1
So far, these peaks are the same as the ones seen for hexane. We can assign them as the C-H stretching and bending frequencies, respectively.
Looking further, you will also see:
- a small peak around 3100 cm-1.
- a small peak near 1650 cm-1.
- medium peaks near 900 and 1000 cm-1.
The peak at 3100 cm-1 hardly seems different from the C-H stretch seen before. It is also a C-H stretch, but from a different type of carbon. This stretch involves the sp2 or trigonal planar carbon of the double bond, whereas the peak at 2900 involves an sp3 or tetrahedral carbon.
The peak at 1650 cm-1 can be identified via computational methods as arising from a carbon-carbon double bond stretch. It is a weak stretch because this bond is not very polar. Sometimes it is obscured by other, larger peaks.
The larger peaks near 800 and 1000 cm-1 are bending vibrations. They are due to a C=C-H bond angle that bends out of the plane of the double bond (remember that the carbons on either end of the double bond are trigonal planar). They are called oop bends. Oop bends are often prominent in alkenes and are easier to spot than an sp2 C-H stretching mode or a C=C stretching mode.
Problem IR4.1.
Given this information about the infrared spectra of alkenes, which bond do you think is stronger, an sp2 or an sp3 C-H bond?
Problem IR4.2.
What do you think would happen to the peak due to carbon-carbon double bond stretching if an electronegative atom were nearby in the molecule?
Sometimes, IR spectroscopy can help to distinguishe some very similar isomers of a compound. In alkenes, Z- and E- isomers have some subtle differences in the IR spectrum. The difference between these isomers has to do with their shapes. In Z-2-octene, the double bond adopts a curled shape with alkyl substituents coming from the same side of the double bond. Z-2-octene is also called cis-2-octene.
![]()
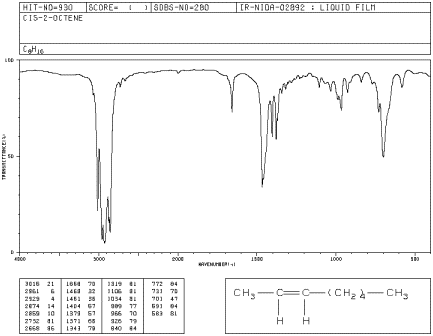
Figure IR4.2. IR spectrum of Z-2-octene.1
In E-2-octene, the double bond adopts a zig-zag shape with alkyl substituents coming from the opposite sides of the double bond. E-2-octene is also called trans-2-octene.
![]()
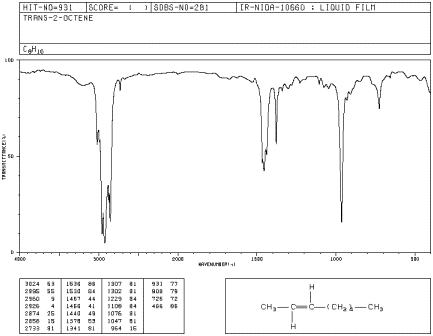
Figure IR4.3. IR spectrum of E-2octene.1
Problem IR4.3.
Oop bends can be diagnostic of the position and geometry of double bonds.
a) Compare the oop bending modes or peaks seen in 1-heptene to those in Z-2-octene, aka cis-2-octene
b) Also compare it to E-2-octene, aka trans-2-octene (in which the double bond has a zig-zag shape, with alkyl substituents coming from opposite sides).
c) Make predictions about the oop bending modes in 1-octene, Z-2-hexene and E-2-hexene.
![]()
![]()
![]()
Problem IR4.4.
Why do you think an sp3 CH2 bending mode occurs around 1500 cm-1 but a C=CH oop bending mode occurs around 800-1000 cm-1 ?
Alkynes are related to alkenes. They contain carbon-carbon triple bonds, whereas alkenes contain carbon-carbon double bonds. This difference leads to some changes in the infrared spectrum. In the IR spectrum of 1-octyne, new peaks appear at 3300 and 2100 cm-1.
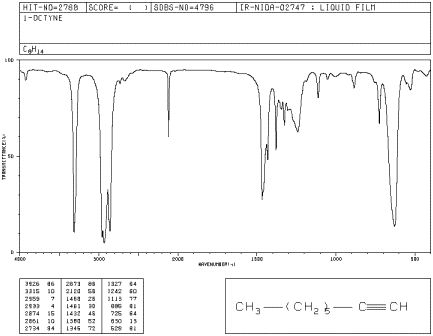
Figure IR4.4. IR spectrum of octyne.1
Problem IR4.5.
a) By comparison with the other hydrocarbons, can you identify the peak at 3300 cm-1 ?
b) The peak at 2100 cm-1 is due to a carbon-carbon bond. Which one? Compare this peak to the one seen from a carbon-carbon bond in 1-hexene and explain the differences.
Ref. 1. SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
