
Structure & Reactivity
Introductory Mass Spectrometry
MS3. GC-MS and LC-MS
There are other aspects of this experiment that have been left out of this description. For example, the experiment is done in a vacuum with a low concentration of sample. Among other reasons, these steps are taken to lower the population of molecules in the instrument; that decreases the chance that ions and molecules will bump into each other and undergo reactions with each other, which might lead to the formation of larger ions with all sorts of different masses.
However, there is one important instrumental consideration to discuss. In mass spectrometry, the sample can be purified immediately after it is introduced into the instrument to make sure that only one kind of molecule is being analysed at a time. This purification is accomplished through chromatography, and so mass spectrometry when done in this way is often referred to as "GC-MS" or "LC-MS", depending on the type of chromatography used.
GC-MS employs gas chromatography, sending components through a chromatography column that is heated in an oven so that compounds can be separated out largely based on their boiling points. LC-MS uses liquid chromatography, in which components are most often separated because of polarity differences, although other criteria can be utilized as well, based on the type of packing material that is used in the chromatography column.
The other advantage of this approach is that several different components of one mixture can be analysed one by one.
The GC part of a GC-MS, termed the chromatograph, generates a plot called a chromatogram.

Figure MS3. Approximate schematic of a GC-MS system, with representative data.
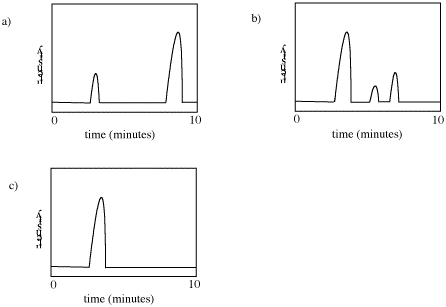
Retention time refers to how long it takes different compounds to emerge from the chromatography column. Peaks are seen on this plot whenever a compound enters the mass spectrometer. The mass spectrometer runs continuously and records whatever masses it detects at any time, so if a student selects a peak in the chromatogram on the computer screen, the corresponding mass spectrum can be shown.
Problem MS3.1. How many compounds appear to be present in each of the following gas chromatograms?
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.Navigation: