NMR12. Constructing Partial Structures in NMR Spectroscopy
When you look at an IR spectrum, you immediately see little chunks of the structure, because you see individual bonds. You know at a glance that the compound contains a C=O bond or an O-H bond. That can be very reassuring, because you can quickly imagine what you are dealing with.
NMR spectra often take more work. You may have to put pencil to paper to come up with a structure. The work pays off, because you can get a much more detailed picture of the structure.
Let's start with a 13C NMR spectrum. Suppose you had peaks in the spectrum at 200, 35, 30, and 15 ppm. We might assign these three peaks as follows:
Table NMR12.1. An example of a data table for 13C NMR.
| shift (ppm) | partial structure |
| 200 | sp2 C=O |
| 35 | sp3 C-C=O |
| 30 | sp3 C-C=O |
| 15 | sp3 C-C |
We are saying that the first carbon is in the sp2 or trigonal planar region, and that it is so far downfield because of a double bond to oxygen. The other two carbons are in the sp3 or tetrahedral region. One of them isn't very far downfield; it is probably just attached to another sp3 carbon. The other two, at 35 and 30 ppm, are both a little further downfield. That's around the right place for a tetrahedral carbon attached to a trigonal planar carbon; that is, these carbons are each attached to either a double bond or a carbonyl.
In the partial structure, we always bold or underline the carbon that corresponds to the peak we are discussing. If you don't do that, it isn't clear whether the peak at 30 comes from a carbon next to the carbonyl (C=O), or the carbon in the carbonyl itself. Also, on the peak at 15, we want to make clear that we are talking about a single carbon atom; leaving the partial structure as C-C somehow implies that this spectroscopy observes bonds, but it doesn't. IR spectroscopy observes bonds. 13C NMR spectroscopy observes carbon atoms.
Now, suppose we look at the 1H NMR spectrum for the same compound. Maybe we see three peaks this time. There is a quartet integrating for 2H at 2.3 ppm, a singlet integrating for 3H at 2.1 ppm, and a triplet integrating for 3H at 1.1 ppm. We enter those characteristics in a table. This time, there are three features to explain for each peak.
Table NMR12.2. An example of a data table for 1H NMR.
| shift | integ. | multipl. | partial structure |
| 2.3 | 2H | quartet | CH3-CH2-C=O |
| 2.1 | 3H | singlet | CH3-C=O |
| 1.1 | 3H | triplet | CH3-CH2 |
First of all, we need to explain the shift. All of these peaks are in the upfield end of the spectrum (below 5 ppm), so they are likely from hydrogens on sp3 or tetrahedral carbons. The first two are slightly downfield, just past 2 ppm. That suggests that the sp3 carbons they are attached to may in turn be attached to sp2 carbons: either double bonds or carbonyls. We already know there is a carbonyl from the 13C spectrum, so let's assume that's what is causing the shift near 2 ppm. The third peak, at 1.1 ppm, is in the normal range; this hydrogen is on a tetrahedral carbon, likely attached to other tetrahedral carbons.
To demonstrate what the integration is telling us, we just show the correct number of hydrogens. There are two hydrogens responsible for the peak at 2.3 ppm. Three others are responsible for the peak at 2.1 ppm, and another three give rise to the peak at 1.1 ppm.
Finally, we need to explain the muliplicity. The peak at 2.3 ppm is a quartet, so by the "n+1" rule it must be next to a CH3 group. The peak at 1.1 ppm is a triplet, so it must be next to a CH2 group. (It doesn't take long to figure out that these two peaks represent hydrogens that are next to each other.) Finally, the peak at 2.1 ppm is a singlet. It has no hydrogen neighbours at all.
Notice we do not need to know what the structure is in order to fill in these partial structures. We are just writing down what the data is telling us. From there, it isn't very far to determine the overall structure.
Problem NMR12.1.
Fill in partial structures for the following peaks.
a) 10.1 ppm, 1H, triplet b) 3.4 ppm, 1H, septet c) 7.3 ppm, 2H, triplet
d) 5.4 ppm, 1H, quartet e) 1.4 ppm, 2H, sextet f) 8.0 ppm, 1H, singlet
g) 2.1 ppm, 3H, singlet h) 6.8 ppm, 2H, doublet i) 0.9 ppm, 6H, doublet
Problem NMR12.2.
Identify the errors in the following partial structures:
a) 3.6 ppm, 2H, triplet, CH2-CH2 b) 2.1 ppm, 2H, singlet, CH3-C=C
c) 7.4 ppm, 2H, doublet, CH=CH2-C d) 1.8 ppm, 2H, quintet, CH2-CH4
e) 7.8 ppm, 1H, triplet, -CH=CH2 f) 1.7 ppm, 1H, nonet, NH2-CH(CH3)2
Once we have worked out partial structures, putting the entire structure together is like putting a jigsaw puzzle together. We just need to find which pieces fit together. The partial stucture shows what each group is attached to, so we can use that information to put things together. Using the information in the complete proton table shown above, we first choose one entry and re-write it where we are going to build up the structure. Put a check or an X beside the entry in the table as a reminder that we have already put this piece in the final structure.
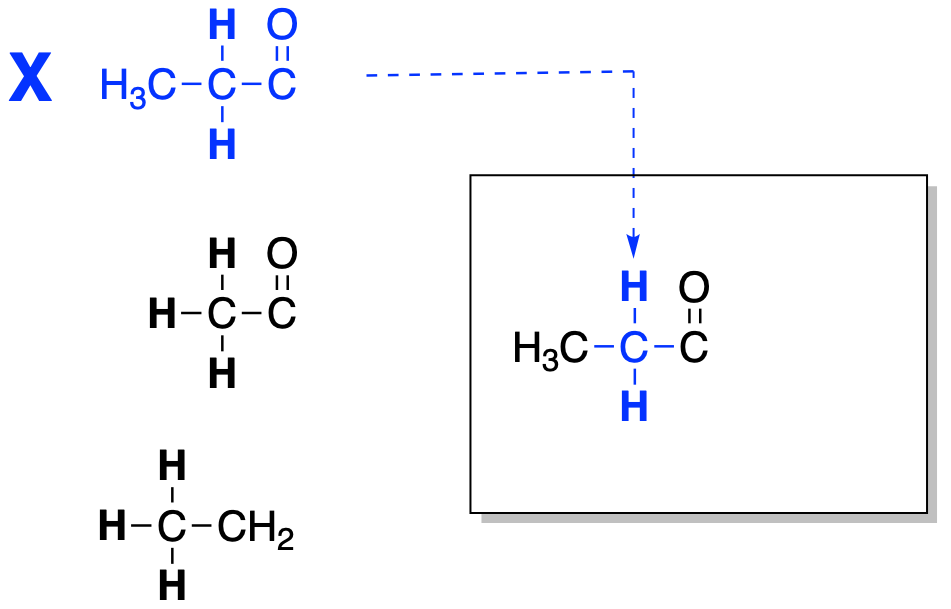
Figure NMR12.1. Fitting together two partial structures to make a bigger structure.
Look at the group that the first unit is attached to. In a partial structure, that's already shown, because the partial structure shows both a set of hydrogens and the hydrogens next to them. In this case, that's the CH3 attached to the CH2. Locate that CH3 group in the table. Mark it off because we already have it in the structure.
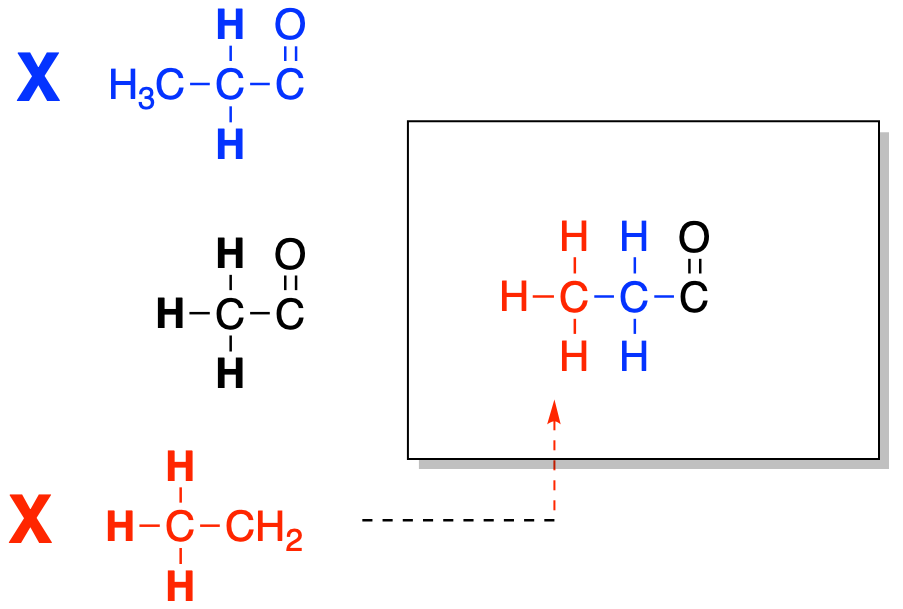
Figure NMR12.2. Fitting together another partial structure to make a bigger structure.
Sometimes, that next piece will show us another attachment, but this time we have reaches the end of the chain. This carbon can't form any more bonds. However, if we look at the right hand side of our structure, we see a carbonyl that still has room for one more bond. We can look in the table and see whether there is a sign of a carbonyl attached to anything else. Once we bring that piece in, the structure is complete.
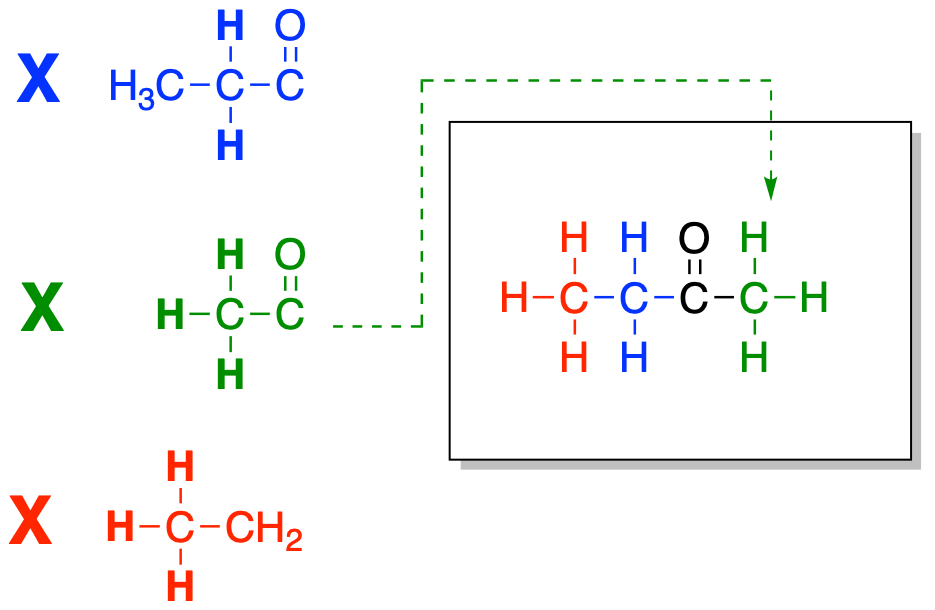
Figure NMR12.3. Fitting together another partial structure to finish building the structure.
Let's try another example. Suppose we have the four partial structures shown below. Like before, we will select a partial structure from our list of partial structures and place it where we want to build the entire structure.
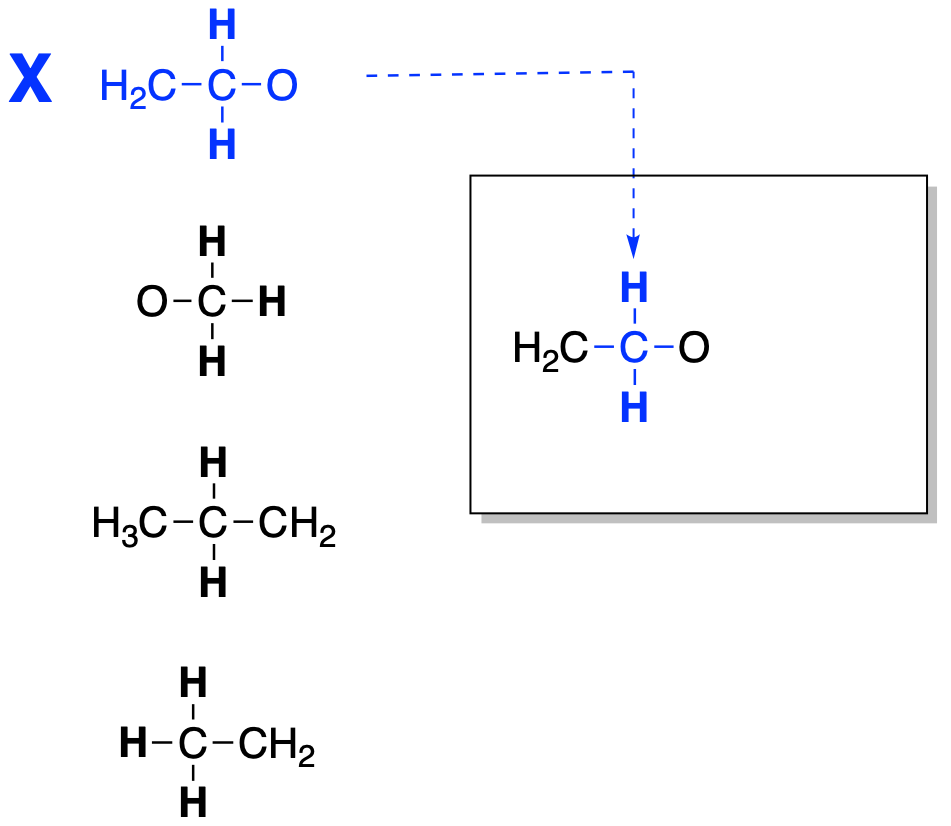
Figure NMR12.4. Fitting together two partial structures to make a bigger structure.
Next, we find the piece that it is connected to and add that one into the structure. We are looking for a second CH2 group. We will place that piece into the structure as well.
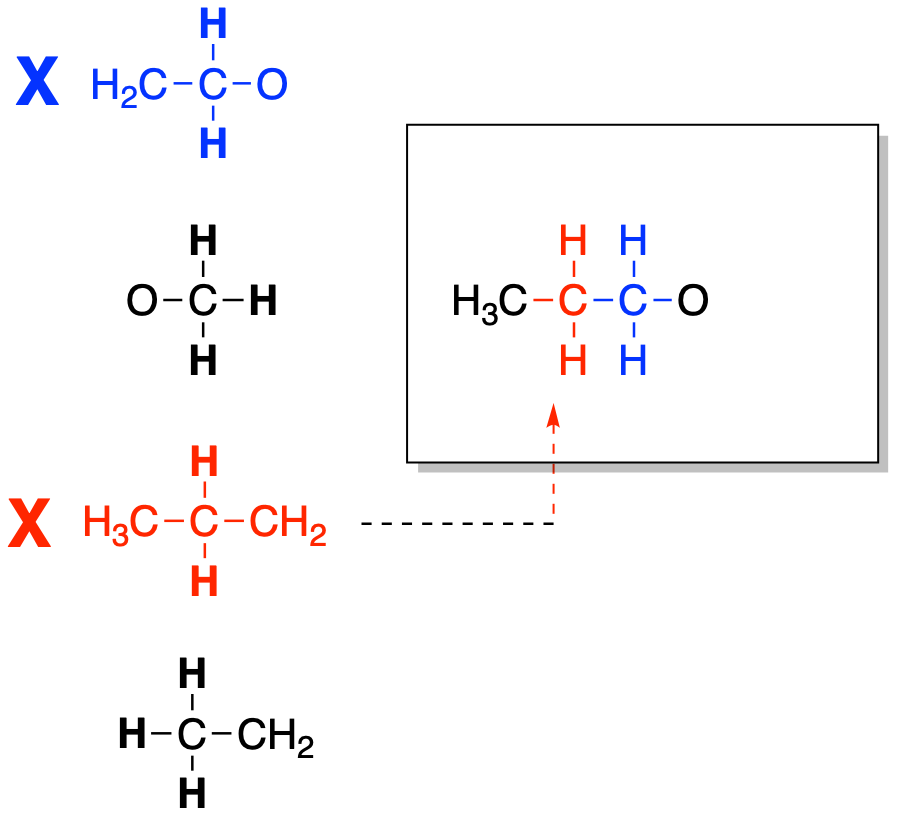
Figure NMR12.5. Fitting together another partial structure to make a bigger structure.
At this point, we have reached the end of the chain on the left side. We need to see whether there is anything else connected to the oxygen and bring that piece into the structure.
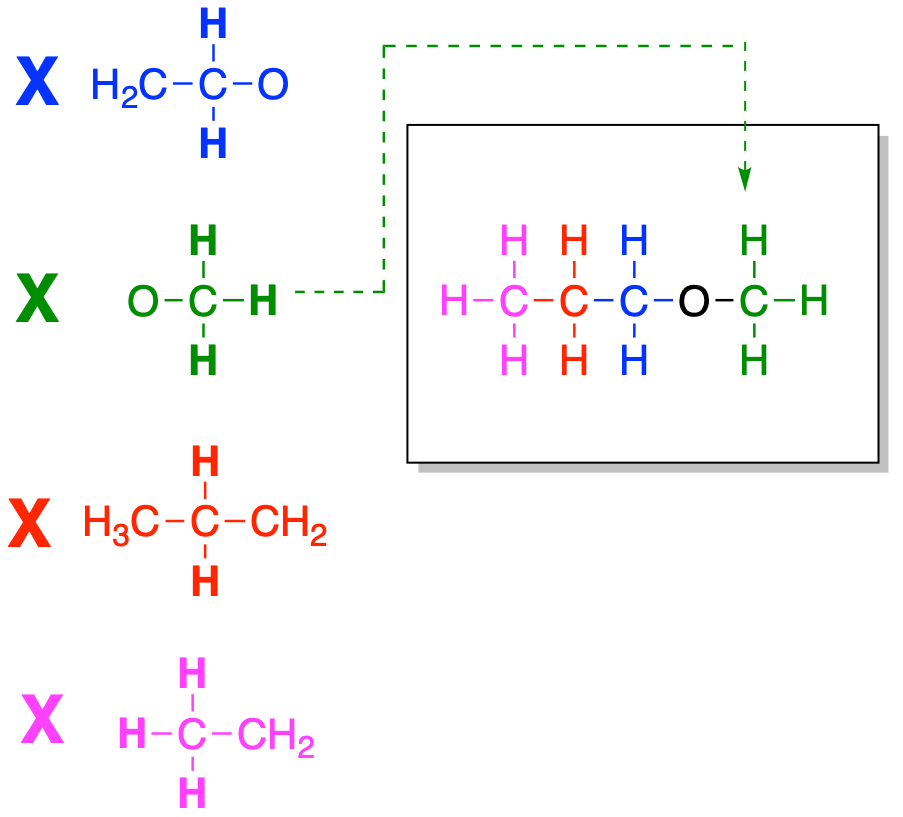
Figure NMR12.6. Fitting together another partial structure to finish building the structure.
Problem NMR12.3.
Put the partial structures together to make the whole structure.
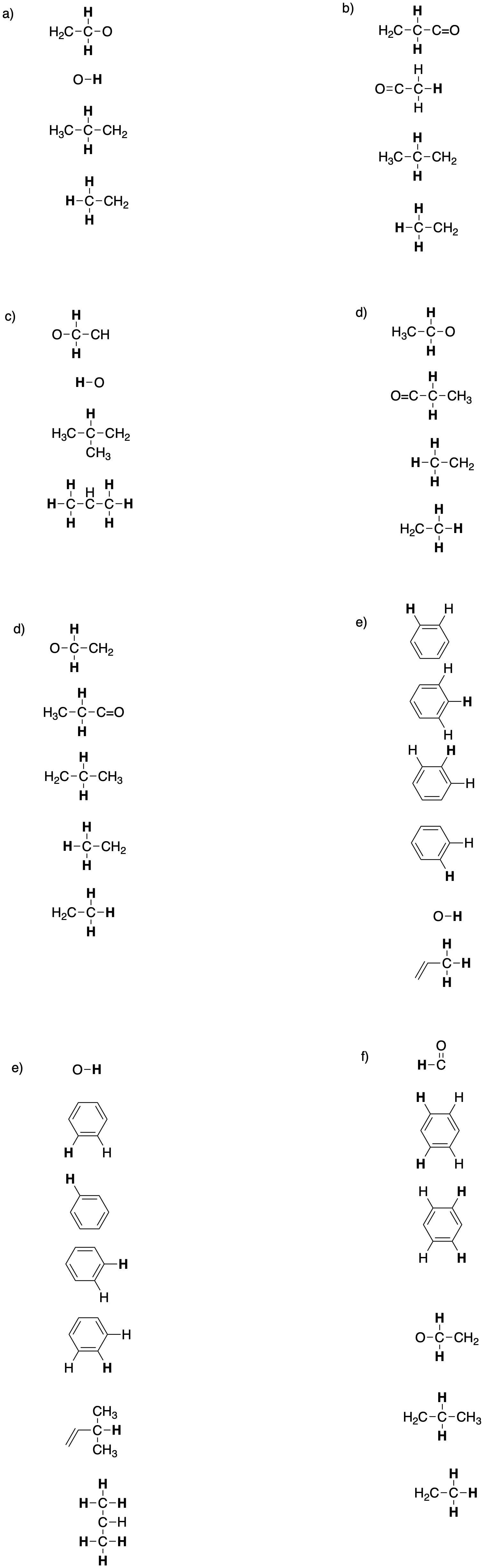
Sometimes aromatic partial structures need to be written in a table using text, rather than drawn. In that case, the partial structure still needs to explain the shift, multiplicity, and integration of the peak. The table below shows an example of how to do that.
| shift (ppm) | integ. | multipl. | partial structure |
| 10.2 | 1H | s | H-C=O |
| 7.8 | 1H | d | CH=CH Ar |
| 7.5 | 1H | t | CH-CH=CH Ar |
| 7.3 | 1H | t | CH-CH=CH Ar |
| 7.2 | 1H | d | CH=CH Ar |
| 2.6 | 3H | s | CH3-C= |
There are a few things to note. In several cases, the reason for the shift is indicated by the double bond to the carbon that bears the hydrogen, suggesting an sp2 carbon rather than the usual sp3 carbons we saw earlier. In one case, it's actually a hydrogen attached to a carbonyl, so the additional downfield shift is indicated by showing the double bond to an oxygen. In other cases, there is an Ar, for aromatic, to further distinguish the shift between the regular alkene region (normally 5-6 ppm) and the aromatic region (usually 6-8 ppm), although there can be overlap between the two regions. The multiplicity is still explained by showing the neighbouring hydrogens, like before, and the hydrogen in bold indicates the hydrogen responsible for the peak in that row of the table, as distinguished from its neighbour. Most of these integrations are for 1H, except for the last one, which is shown as CH3. However, if one of the aromatic peaks had integrated to 2 H, we could not have shown something like "HC-CH2=CH Ar" for a triplet, for example, because that would make five bonds to carbon. In general, an aromatic carbon can have only one hydrogen attached. In that case, we would need an additional note, so we would show something like "HC-CH=CH Ar x2", in which the x2 is indicating symmetry, so that there are actually two identical positions like this. Also, in the very last row, the shift could have been produced by the hydrogen being on an sp3 carbon attached to either a C=C or a C=O, so the other end of the double bond was left undefined.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: