Structure & Reactivity
Nuclear Magnetic Resonance Spectroscopy
NMR2D.7. 2D NMR Solutions
All problems are contributions from Edward McIntee and Kate Graham, College of Saint Benedict | Saint John's University
Problem NMR2D1.1.
ethyl butanoate
Problem NMR2D1.2.

Problem NMR2D1.3.
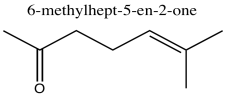
Problem NMR2D1.4.
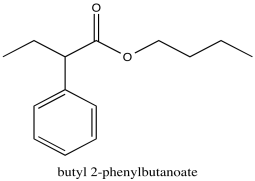
Problem NMR2D1.5.
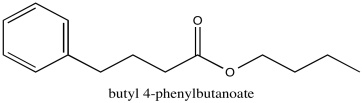
Problem NMR2D1.6.
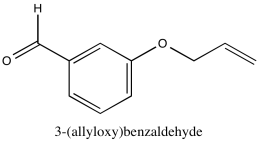
Problem NMR2D1.7.
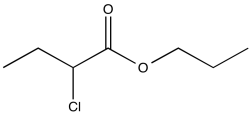
Problem NMR2D1.8.
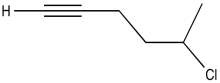
Problem NMR2D1.9.
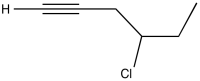
Problem NMR2D1.10.
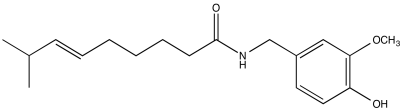
Problem NMR2D1.11.
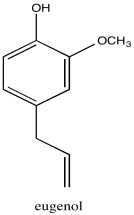
Problem NMR2D1.12.
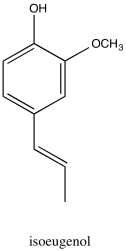
Problem NMR2D2.1.
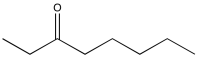
Problem NMR2D2.2.
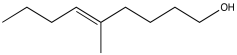
Problem NMR2D2.3.
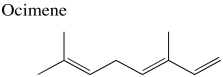
Problem NMR2D3.1.
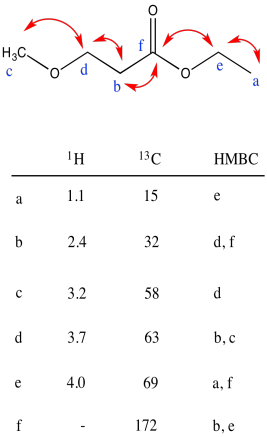
Problem NMR2D3.2.
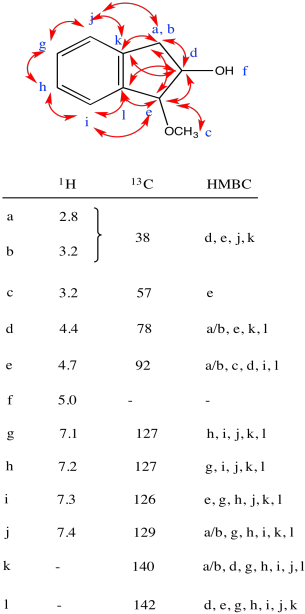
Problem NMR2D3.3.
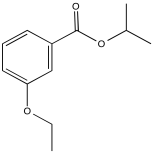
Problem NMR2D3.4.
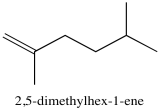
Problem NMR2D3.5.
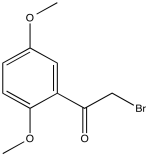
Problem NMR2D3.6.
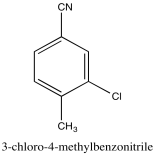
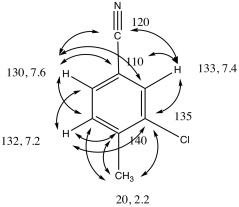
Problem NMR2D3.7.
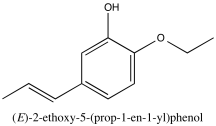
Problem NMR2D3.8.
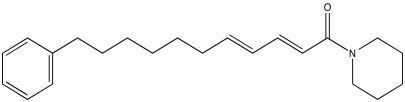
Problem NMR2D.4.1.
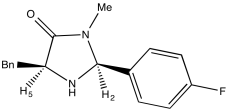
Problem NMR2D.4.2.
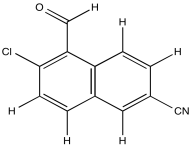
Problem NMR2D.4.3.
a) Due to resonance, there is substantial pi character to the amide bond which restricts free rotation around that bond.
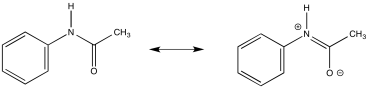
b)
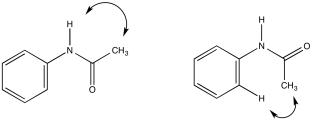
c)
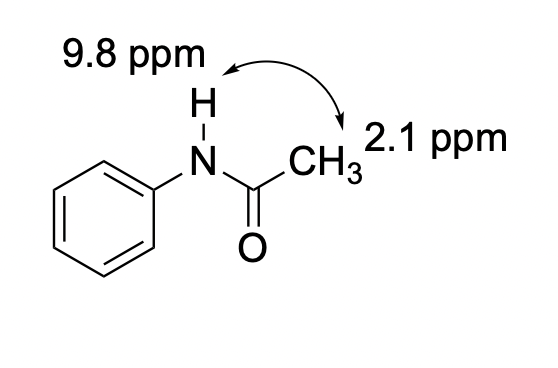
Problem NMR2D.4.4.
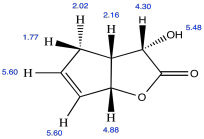
Problem NMR2D.4.5.
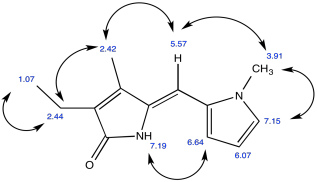
Problem NMR2D.4.6.
a)
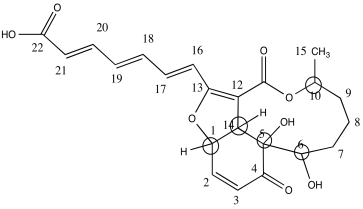
b)
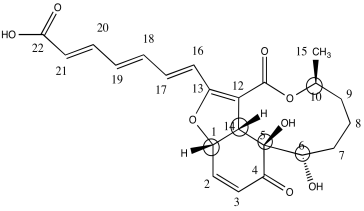
c)
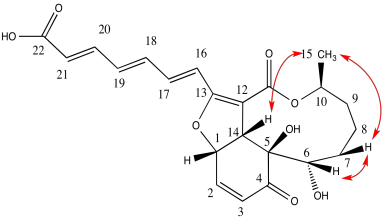
Problem NMR2D.4.7.
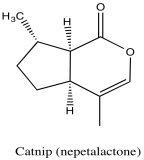
Problem NMR2D5.1.
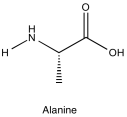
Problem NMR2D5.2.
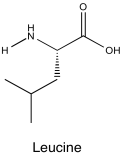
Problem NMR2D5.3.
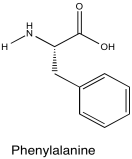
Problem NMR2D5.4.
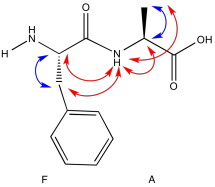
Problem NMR2D5.5.
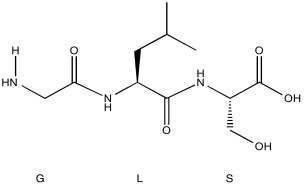
Problem NMR2D6.1.
1H NMR
| Chemical shift (ppm) | Integration | Multiplicity | Partial Structure |
| 5.54 | 2H | multiplet | CH=C (x 2) |
| 4.17 | 2H | doublet | O-CH2-CH |
| 2.08 | 2H | quintet | CH-CH2-CH3 |
| 1.47 | 1H | boad singlet | OH |
| 0.95 | 3H | triplet | CH2-CH3 |
* total # H: 10
13C NMR
|
Chemical shift (ppm) |
Type of carbon |
| 134 | sp2 |
| 128 |
sp2 |
| 58 | sp3-O |
| 21 | sp3 |
| 14 | sp3 |
*total # C: 5
COSY
| Assignment | 1H | COSY |
| A | 0.95 | 2.08 |
| B | 2.08 | 0.95, 5.54 |
| C | 4.17 | 5.54 |
| D | 5.54 | 2.08 |
| E | 5.54 | 4.17 |
*HMQC indicates two hydrogens at 5.54 are in two different environments
HMQC
| Assignment | 13C | 1H |
| A | 14 | 0.95 |
| B | 21 | 2.08 |
| C | 58 | 4.17 |
| D | 128 | 5.54 |
| E | 134 | 5.54 |
Formula:
C5H10O (1 O indicated from shift in 13C, 1H NMR)
FW = (5 x 12) + (10 x 1) + 1 x 16) = 86
Compare C5H10 ratio to C5H12 in hydrocarbon
Degrees of unsaturation = [(2 x 5) + 2 - 10] / 2 = 1 unit (1 double bond)
The data tables should be consitent with this structure:
pent-2-en-1-ol (could be cis or trans based on this analysis)
Problem NMR2D6.2.
1H NMR:
| Chemical shift (ppm) | Integration | Multiplicity | Partial structure |
| 4.7 | 5H | singlet | solvent |
| 3.93 | 1H | triplet | CH2-CH-N |
| 2.40 | 2H | multiplet | CH2-CH2? |
| 2.09 | 2H | multiplet | CH2-CH2? |
| 1.4 | 9H | singlet | C(CH3)3 |
*Total number of H: 19 H
13C NMR:
| Chemical shift (ppm) | Type of carbon |
| 170 | sp2 (C=O) |
| 80 | sp3 (C-O) |
| 52 | sp3 (C-N) |
| 32 | sp3 |
| 28 | sp3 |
| 26 | sp3 |
*Total number of C: 6 apparent, but two more suggested by symmetry (3 methyl groups in 1H NMR) for 8 C; a third extra suggested by MW fit for 9 C
COSY:
| Assignment | 1H | COSY |
| Solvent | 4.7 | -- |
| B | 3.93 | 2.40 |
| D | 2.40 | 2.09 |
| C | 2.09 | 2.40, 2.09 |
| A | 1.4 | -- |
Formula:
C9H18O3N2 (extra O indicated from shift in 13C, 1H NMR; second O suggested by C=O in 13C NMR; additional CO needed to fit MW)
FW = (9 x 12) + (18 x 1) + (3 x 16) + (2 x 14) = 202
FW = (9 x 12) + (18 x 1) + (3 x 16) + (2 x 14) = 202
Compare C9H18 to C9H22 for the corresponding hydrocarbon corrected for two nitrogens (therefore two extra hydrogens)
Degrees of unsaturation = [(2 x 9) + 2 + 2 - 10] / 2 = 2 units (2 double bonds)
The data tables should be consistent with this structure:
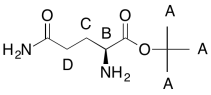
Problem NMR2D6.3.
The data should be consistent with this structure:
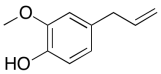
Problem NMR2D6.4.
1H NMR:
|
Chemical shift (ppm) |
Integration | Multiplicity | Partial Structure |
| 4.71 | -- | singlet | solvent |
| 4.17 | 1H | doublet? | CO-CH-N |
| 3.75 | 3H | singlet | O-CH3 |
| 2.25 | 1H | multiplet | CH-CH-(CH3)2 |
| 0.92 | 6H | triplet? | 2 x CH3 |
13C NMR:
| Chemical shift (ppm) | Type of carbon |
| 170 | sp2 C=O |
| 60 | sp3 C-N |
| 52 | sp3 C-O |
| 30 | sp3 C |
| 19 | sp3 C |
COSY:
| Assignment | 1H | COSY |
| A | 4.17 | 2.25 |
| B | 3.75 | -- |
| C | 2.25 | 4.17 |
| D | 0.92 | 2.25 |
Formula:
C6H13O2N (1 O indicated from shift in 13C, 1H NMR)
FW = (6 x 12) + (13 x 1) + (2 x 16) + (1 x 14) = 131
Degrees of unsaturation = [(2 x 6) + 2 + 1 - 13] / 2 = 1 unit (1 double bond)
The data should be consistent with this structure:
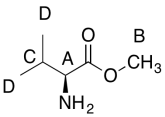
Problem NMR2D6.5.
The data should be consistent with this structure:
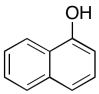
Problem NMR2D6.6.
The data should be consistent with this structure:
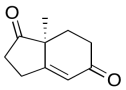
Problem NMR2D6.7.
The data should be consistent with this structure:
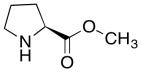
Problem NMR2D6.8.
The data should be consistent with this structure:
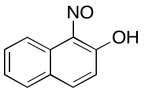
Problem NMR2D6.9.
The data should be consistent with this structure:
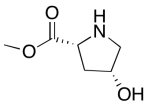
Problem NMR2D6.10.
The data should be consistent with this structure:
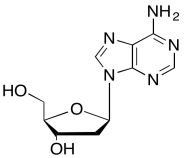
Problem NMR2D6.11.
The data should be consistent with this structure:
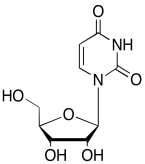
Problem NMR2D6.12.
The data should be consistent with this structure:
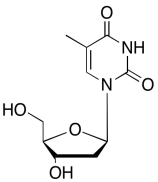
Problem NMR2D6.13.
1H NMR:
| Chemical shift (ppm) | Integration | Multiplicity | Partial structure |
| 5.7 | 1H | singlet | C=CH-CO |
| 2.77 | 1H | multiplet | CH2-CH-CO |
| 2.62 | 1H | multiplet | C-CH-C |
| 2.39 | 1H | multiplet | C-CH-C |
| 2.05 | 1H | doublet? | C-CH-CH? |
| 1.96 | 3H | singlet | C-CH3 |
| 1.48 | 3H | singlet | C-CH3 |
| 0.98 | 3H | singlet | C-CH3 |
13C NMR:
| Chemical shft (ppm) | Type of carbon |
| 204 | sp2 C=O |
| 170 | sp2 |
| 121 | sp2 |
| 59 | sp3 |
| 55 | sp3 |
| 50 | sp3 |
| 41 | sp3 |
| 28 | sp3 |
| 24 | sp3 |
| 22 | sp3 |
COSY:
| Assignment | 1H | COSY |
| 1 | 2.39 | 2.77, 2.62? |
| 3 | 5.7 | 2.05? |
| 5 | 2.62 | 2.62? |
| 7a | 2.77 | 2.05 |
| 7b | 2.05 | 2.77 |
| 8 | 1.48 | -- |
| 9 | 0.98 | -- |
| 10 | 1.96 | -- |
Formula:
C10H14O (1 O indicated from shift in 13C, 1H NMR)
FW = (10 x 12) + (14 x 1) + (1 x 16) = 150
Degrees of unsaturation = [(2 x 10) + 2 - 14] / 2 = 4 units (e.g. 2 rings, 2 double bonds)
The data should be consistent with this structure:
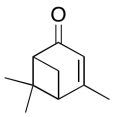
Problem NMR2D6.14.
The data should be consistent with this structure:
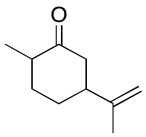
Problem NMR2D6.15.
The data should be consistent with this structure:
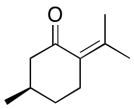
All problems are contributions from Edward McIntee and Kate Graham, College of Saint Benedict | Saint John's University
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Useful Charts for NMR identification.