Reactivity in Chemistry
Mechanisms of Glycolysis
GL7. Catalysis of Phase Two
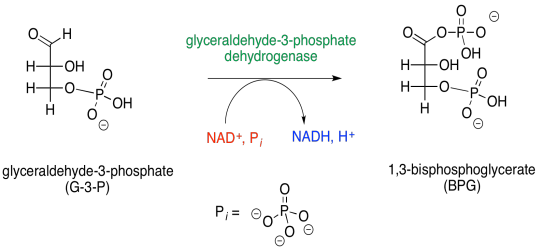
In phase two of glycolysis, glyceraldehyde-3-phosphate proceeds through an oxidation reaction, in which an aldehyde hydrogen is replaced by a phosphate group. If you are scratching your head, wondering how that reaction might happen, then that's a good thing. The hydrogen is not a good leaving group, and you haven't seen much precedent for this kind of reaction.

Figure GL7.1. Step 1: G3P is converted to BPG.
This step looks like a very good place for catalysis. The reaction doesn't look like it will work very well, and so we need an alternative, lower-energy pathway. At the very least, this is a good situation for the enzymatic strategy of approximation, in which two substrates are held near each other by their respective placements in the active site. Approximation is needed because of that poor hydride leaving group, H-. It really can't be displaced from the molecule to become a hydride ion in free solution, because it isn't stable enough. It needs that NAD+ acceptor to be waiting for it as it comes off the molecule of glyceraldehyde-3-phosphate.
But the hydride isn't displaced by the phosphate directly; there is an invisible step in between. A cysteine residue from the enzyme first donates to the carbonyl. Sulfur is a very good nucleophile for carbon electrophiles; sulfur is a little less electronegative and more polarisable than oxygen, so it can donate electrons relatively easily.
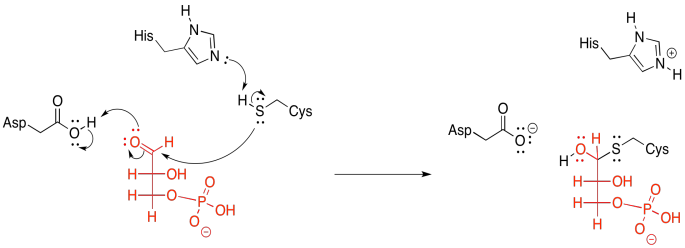
Figure GL7.2. G3P is converted to a hemithioacetal.
That donation results in the formation of a hemithioacetal. In addition, it tethers the glyceraldehyde molecule to the enzyme. This feature is an example of the group transfer strategy, in which the substrate becomes temporarily attached to the enzyme that is working on it. This group transfer might be explained as gaining an entropic edge to help get through an enthalpically difficult step. This next part may be a little bit tricky, so let's just tie the substrate down for a moment.
So, now we are ready to pi-donate from the tetrahedral intermediate, pushing the hydride onto the waiting NAD+, like a fast pitch straight into the catcher's mitt.
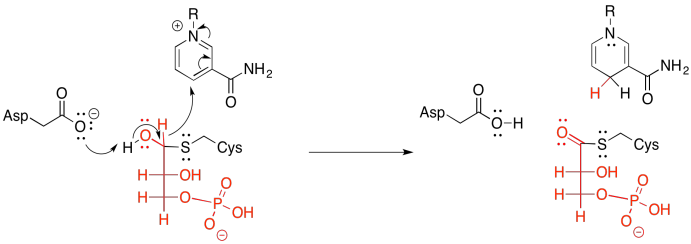
Figure GL7.3. The thioacetal is converted to a thioester.
Of course, group transfer always relies on the substrate being cut from the enzyme again. Otherwise, the transfer would be an example of irreversible inhibition; the substrate molecule would be stuck in the enzyme, forever blocking its active site. The thing about sulfur, though, is that its polarisability makes it a pretty good leaving group. Remember, too, that thioesters are more reactive than regular esters. Thioesters sit higher on the ski hill illustrating the relative reactivity of carboxyloids. That means that the use of a cysteine residue as the tethering group, rather than a lysine's nitrogen or a serine's oxygen, is particularly advantageous for the cleavage step.
The cleavage step involves donation from an inorganic phosphate ion. The carbonyl opens up to give a tetrahedral intermediate, and when that intermediate collapses again, the cysteine is released.
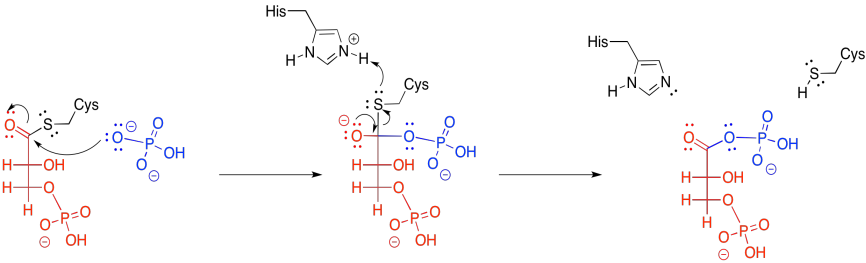
Figure GL7.4. The thioester is converted to a phosphoanhydride.
The second step of phase two looks a little simpler. It's just a displacement of a carboxylate leaving group from a phosphoanhydride.
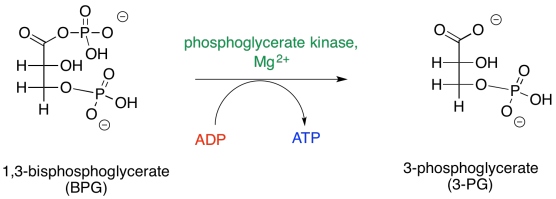
Figure GL7.5. Step 2: BPG is converted to 3PG.
Remember, like the thioester it was formed from, this anhydride was high on the ski hill, because the carboxylate anion is a good leaving group. The phosphate donates to the phosphoryl group, resulting in a five-coordinate phosphorus. This intermediate collapses, ejecting the carboxylate leaving group.
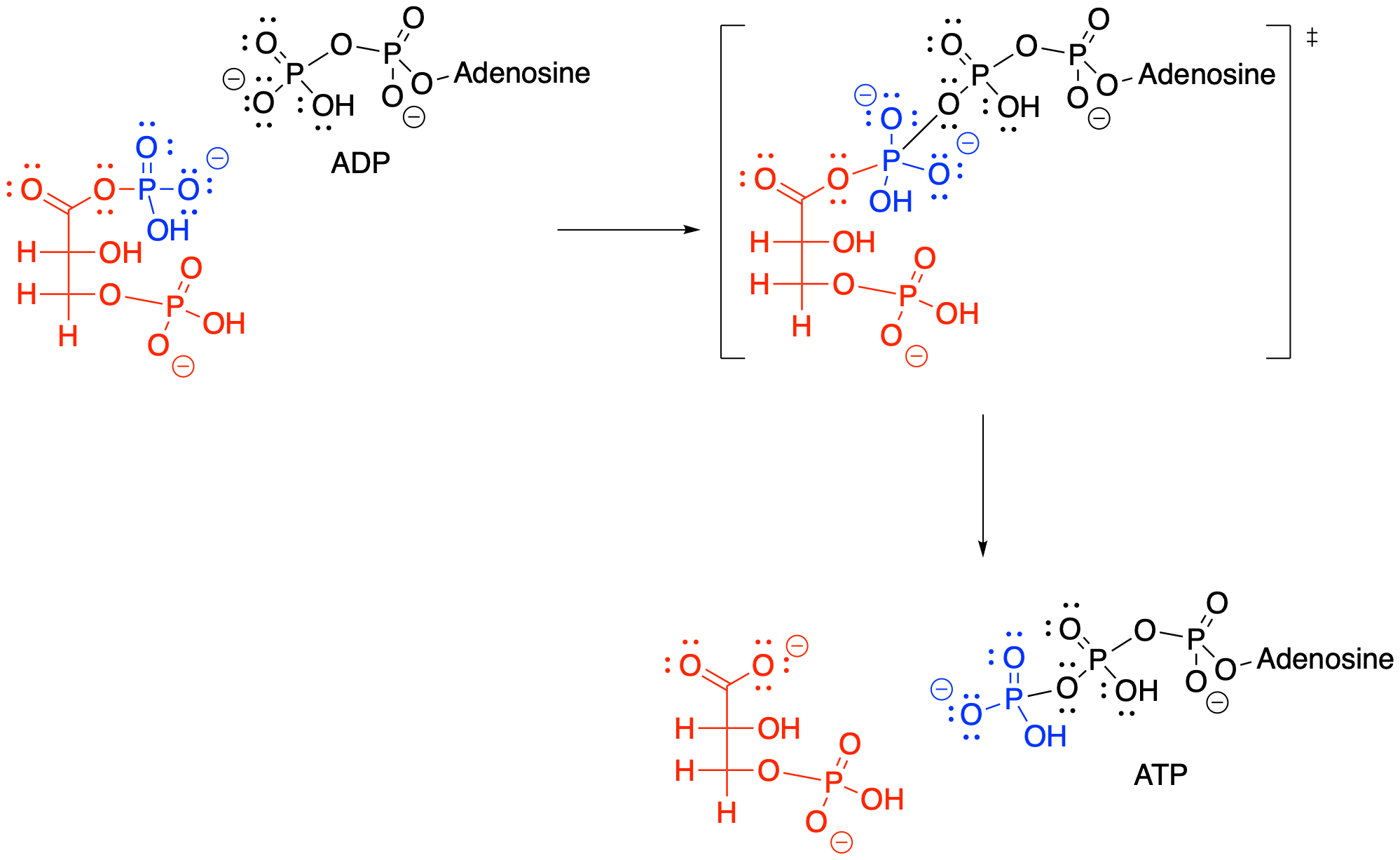
Figure GL7.6. Step 6: ADP acts as a nucleophile to BPG, making ATP and 3PG.
The third step also appears straightforward. It looks like the phosphate is just moving from one oxygen to another.

Figure GL7.7. Step 3: 3PG is converted to 2PG.
In practice, things are slightly more complicated. In animals, the phosphate that comes from the 3-position isn't the same as the phosphate that ends up at the 2-position. In other words, rather than just moving the phosphate from one place to another, a new phosphate is added before the old one is removed.
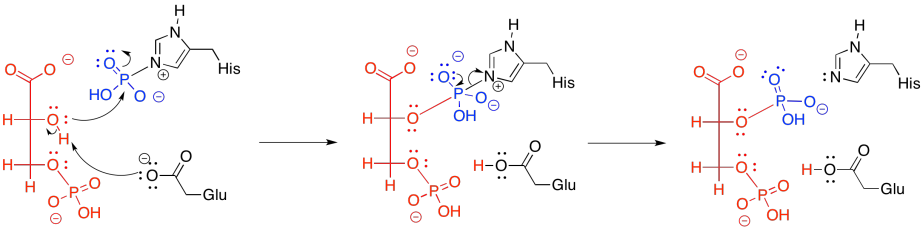
Figure GL7.8. 3PG is phosphorylated and (see GL7.9) selectively dephosphorylated.
The new phosphate is delivered from a modified histidine residue in the enzyme. The same histidine can then pick up the old phosphate from the 3-position, which it will then hold onto, waiting to deliver it to the next substrate molecule that arrives in the enzyme.
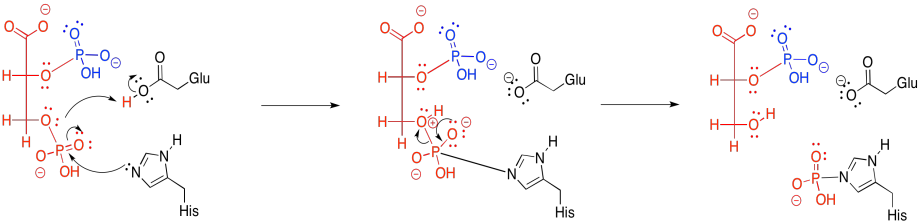
Figure GL7.9. 3PG is phosphorylated (see GL7.8) and selectively dephosphorylated.
The fourth step is, in principle, a simple dehydration.
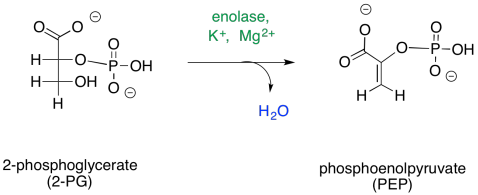
Figure GL7.10. Step 4. 2PG is converted to PEP.
Once again, this step requires a cation to activate it. In some cases, two magnesium ions appear to be involved: one to bind the phosphate and one to bind the carboxylate. Other cases employ a magesium ion and a potassium ion.
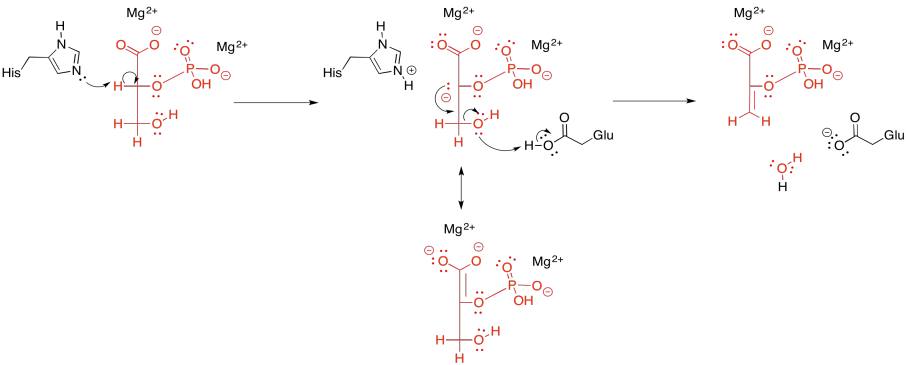
Figure GL7.11. Dehydration of 2PG via an E1CB mechanism.
Either way, coordination to a metal ion probably lowers the pKa of the alpha hydrogen. Instead of deprotonating to produce a tri-anion or tetra-anion (depending whether the phosphate is already singly deprotonated or doubly deprotonated), the metal-bound substrate will produce an anion with overall lower negative charge that we would have otherwise. The deprotonation seems to be carried out by a lysine in some enzymes and a histidine in others.
After that, the beta- hydroxy group is lost. It needs to pick up a proton to become water. That proton may be supplied by a nearby glutamate, which shuttles the proton from elsewhere.
The final step in glycolysis is another phosphate transfer.
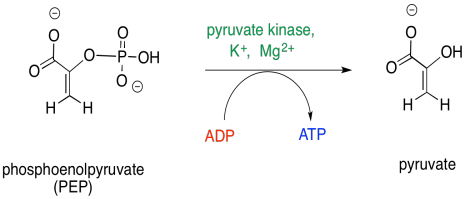
Figure GL7.12. Step 5: PEP is converted to pyruvate.
Once again, ADP is the nucleophile that displaces the leaving group from the phosphate electrophile. This time, the leaving group is an enolate anion. The reaction requires magnesium ions to hold the ADP in place.
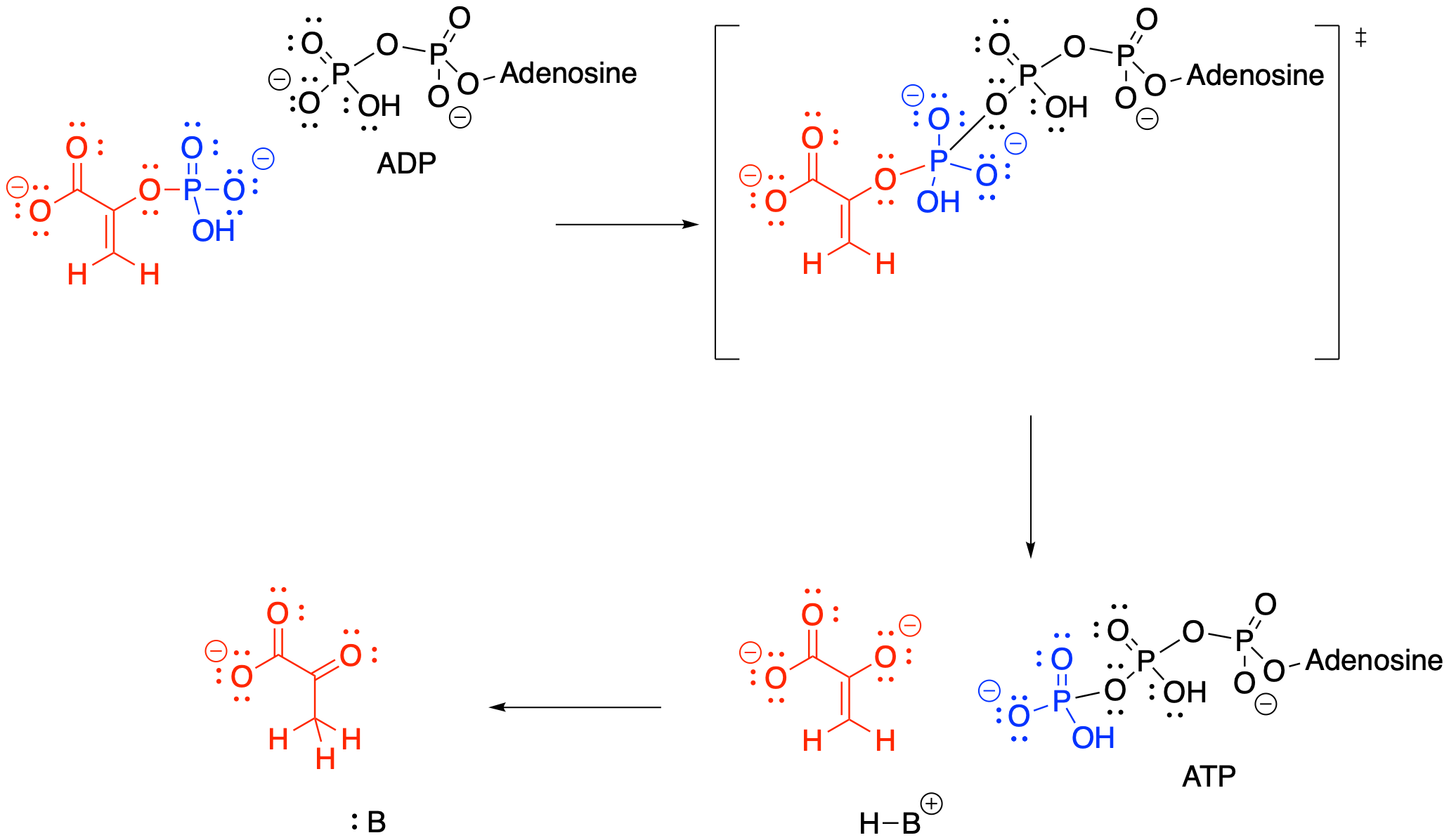
Figure GL7.12. Step 5: ADP is a nucleophile for PEP, making ATP and pyruvate.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: