Reactivity in Chemistry
Mechanisms of Glycolysis
GL3. Mechanisms of Phase One: Phosphorylation and Isomerisation
In the next couple of sections, we will look at the changes occurring in reactions of glycolysis and try to understand how those changes may be happening. In some cases, we will develop a formal understanding of the reaction in a slightly simplified way. We will return in a later section to see how that reaction is actually catalysed by an enzyme.
The first phase of glycolysis is all about taking the initial carbohydrate, glucose, and getting it into the right form for the energy-releasing, ATP-forming reactions of the second phase. All of the reactions of glycolysis are catalysed by enzymes. If you know anything about enzymes, you may know that there are many controls in place so that their reactions can be turned on or off.
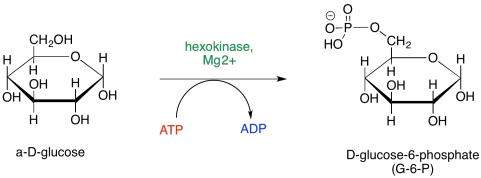
One common element of enzyme control is phosphorylation. In phosphorylation, a phosphate group is added. The phosphate group could be added to the enzyme, or it could be added to the substrate -- the compound on which the enzyme carries out a reaction. If you look again at the map of Phase One, you will see a couple of phosphorylations taking place. Glucose is phosphorylated to start things off, and later on, a fructose phosphate is again phosphorylated to make a fructose bisphosphate.

Figure GL3.1. Phosphorylation of glucose.
Phosphorylation typically modifies the interaction of a substrate with its surroundings. It could be that phosphorylation, and the negative charge that results on the glucose, helps the glucose to bind more tightly with the next enzyme in the pathway. It is also believed that the anionic glucose phosphate is less likely to leave the cell, because it can't be taken up at the nonpolar membrane and transported across the membrane to the outside of the cell. A cell that needs energy would find it advantageous to prevent its glucose from escaping.
Problem GL3.1.
Provide a mechanism for the phosphorylation of glucose.
The second step of glycolysis is an isomerisation. The carbonyl of glucose, on the first carbon in the chain form, migrates to the second carbon in the chain.

Figure GL3.2. Isomerisation of glucose-6-phosphate.
The key to understanding this reaction is noting that, when the second carbon in the chain becomes a carbonyl, it loses a hydrogen.
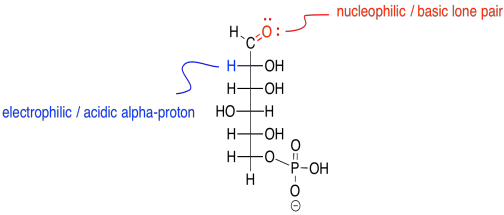
Figure GL3.3. Reactive sites of glucose-6-phosphate.
The reaction actually occurs via an enol intermediate. Remember, an enol is just a tautomer of the original compound; that means it is an isomer in which the major difference is the position of one proton. In an enol tautomer, one proton has been moved from the alpha position, next to the carbonyl, over to the carbonyl oxygen.
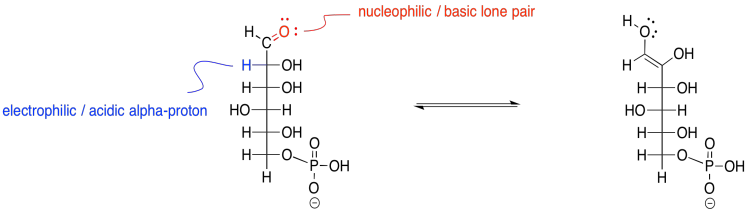
Figure GL3.4. Keto-enol tautomerism to provide an enol intermediate.
Problem GL3.2.
Keto-enol tautomerism involves eventual transfer of a proton from one site to another within the same molecule. The reaction is subject to general catalysis, meaning it can be carried out either by acid or by base.
Provide a mechanism for the conversion of 2-propanone into its enol form in the presence of:
a) aqueous hydrochloric acid
b) aqueous sodium hydroxide
Problem GL3.3.
Provide a mechanism for the keto-enol tautomerism of glucose-6-phosphate. Assume acidic, biological conditions (for example, assisted by the acidic form of a lysine side chain).
Another enol-keto equilibrium is needed in order to complete the overall reaction. This time, it is the enol form going back into a keto form. That means the proton is being transferred from an OH group along the C=C bond (the enol position) back to the alpha position. But take a close look at this molecule. It actually has two different enol OH groups. Either one could lose its proton and turn back into a carbonyl. One of those events leads right back where we started from. The other one leads forward to fructose-6-phosphate.
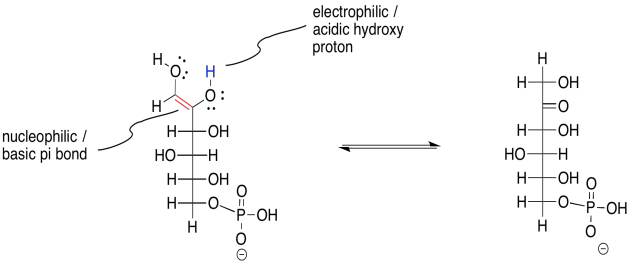
Figure GL3.5. Keto-enol tautomerism completes the reaction to form fructose-6-phosphate.
Why did this step have to take place? Remember, the eventual end product of phase one of glycolysis is a three-carbon sugar, G3P. Right now we have a six carbon sugar (fructose, not glucose, but still a six-carbon sugar). The migration of the carbonyl from the first carbon to the second is what will allow the glucose molecule to break in half, between the third and the fourth carbon. It is going to break in an alpha position, next to a carbonyl. We have just moved the carbonyl to put the alpha position in the right place.
Problem GL3.4.
Provide a mechanism for the keto-enol tautomerism that forms fructose-6-phosphate. Assume basic, biological conditions (for example, assisted by the basic form of a lysine side chain).
Problem GL3.5.
Frequently, formation of an enol from a ketone could result in different products. Show the enol products (there are at east two in each case) that could form from each of the following compounds.
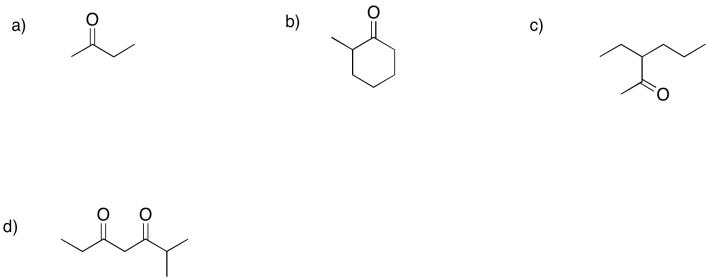
Problem GL3.6.
Each of the following enols could form two different keto tautomers via keto-enol tautomerism. Show both products in each case.
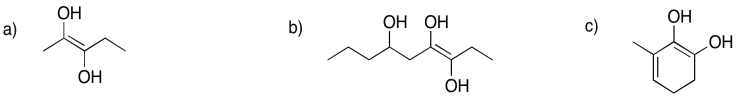
The subsequent step in the pathway is that second phosphorylation, forming fructose-1,6-bisphosphate. The compound changes from an anion to a dianion, and that change has consequences in how the molecule interacts with its environment. Once again, the molecule becomes even less likely to move into the nonpolar cell membrane. Furthermore, it can now interact with different enzymes in a way it couldn't when it was just a monoanion.
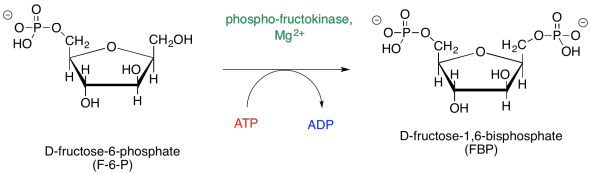
Figure GL3.6. Another phosphorylation to form fructose-1,6-bisphosphate.
This phosphorylation requires the consumption of another molecule of ATP. Remember, this phase of glycolysis is not the energy-producing part. We are still getting ready for that part. It will happen in phase two, but we still have more to do in phase one on the next page.
Problem GL3.7.
Provide a mechanism for the formation of FBP from F6P.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: