Reactivity in Chemistry
Mechanisms of Glycolysis
GL6. Mechanisms of Phase Two
Phase one of glycolysis is setting the stage. An initial investment of ATP is needed to get the system ready to produce more ATP; it's like a farmer who sows sunflower seeds in the spring to get a bigger crop of sunflower seeds in the fall.
Two molecules of glyceraldehyde-3-phosphate are produced in phase one of glycolysis. Both of those molecules enter into phase two. The first step in this phase is an oxidation reaction. In organic chemistry, the term oxidation suggests that a carbon atom is getting fewer bonds to hydrogen, or more bonds to oxygen. (The complementary term, reduction, suggests new carbon-hydrogen bonds are forming, or carbon-oxygen bonds are disappearing.) Notice that the carbonyl carbon, C=O, is becoming a carboxylic carbon, O-C=O.

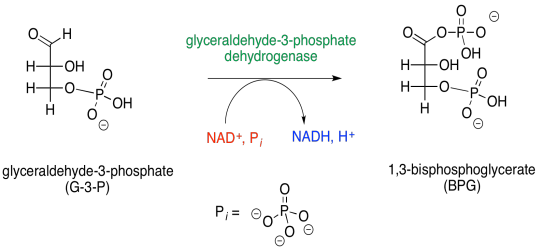
Figure GL6.1. G3P is converted to BPG.
That isn't all that's going on. There is also a phosphorylation step here, but this time ATP is not required. The source of the phosphate group is a simple phosphate ion.
Furthermore, a molecule of NADH is produced. You might remember that NAD+ can pick up a hydride ion (that's right, H- instead of H+) to become NADH. In this case, the hydride ion is coming from the aldehyde that is converted into a carboxyloid, the phosphoric anhydride group in BPG.
This first step has consequences for energy-packaging pathways further downstream. NADH is the starting material for oxidative phosphorylation, an elegant process in which electrons are passed from one metal ion to another within membrane-bound proteins; as the electrons move across the membrane, they draw oppositely-charged protons along with them. A proton gradient builds up, with protons on one side of the membrane outnumbering those on the other; this osmotic pressure is relieved when the protons find a channel to pour back through the membrane, but as they do so they turn a molecular millwheel that drives the production of more ATP. Remember, oxidative phosphorylation, along with glycolysis and the citric acid cycle, is one of the three pathways that together make up the process of respiration.
There is also a more immediate energy-packaging result. That first step produces 1,3-bisphosphoglycerate, which is primed to deliver a phosphate to a molecule of ADP. In addition to ATP, a molecule of 3-phosphoglycerate is left behind.
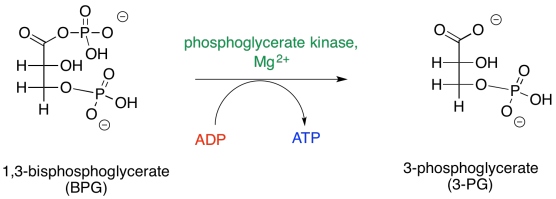
Figure GL6.2. BPG is converted to 3PG.
A slight modification of the 3-phosphoglycerate ensues. In this step, the phosphate group migrates from the 3-position to the 2-position, resulting in 2-phosphoglycerate.

Figure GL6.3. 3PG is converted to 2PG.
Subsequently, the 2-phosphoglycerate undergoes a dehydration, the loss of water. You might recall that dehydrations sometimes occur after aldol reactions: the O=C-CH-C-OH loses a proton at the alpha position and a hydroxide at the beta position to give the enone group, O=C-C=C, and water, HOH. That step is often driven by the conjugated system that results. In this case, the conjugated product is phosphoenolpyruvate.
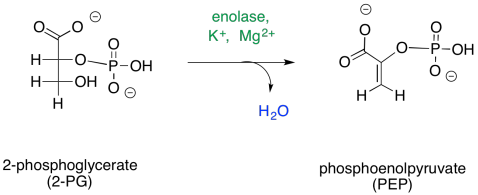
Figure GL6.4. 2PG is converted to PEP.
The final step in glycolysis is the loss of the phosphate group from phosphoenolpyruvate. This phosphate group is transferred to another molecule of ADP, forming ATP. The ATP can then be used to power processes elsewhere in the cell. Note that this is the second molecule of ATP produced during phase two. Since each molecule of glucose produces two three-carbon sugars that enter into phase two, a total of four molecules of ATP are produced per glucose. Remember, phase one required the consumption of two molecules of glucose, so this yield represents a doubling of the initial investment of ATP. It's like putting two dollars in the bank and getting four dollars back out.
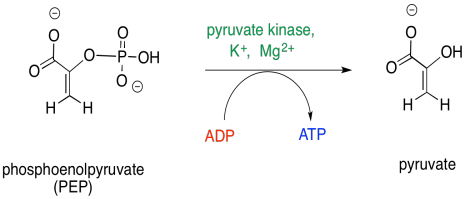
Figure GL6.5. PEP is converted to pyruvate.
That's the end of glycolysis, but that's not the end of the story. So far, glucose has only been broken down to a three-carbon sugar. When we think of respiration, we think of glucose breaking down all the way to carbon dioxide. That part continues in the tricarboxylic acid cycle, or TCA cycle.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: