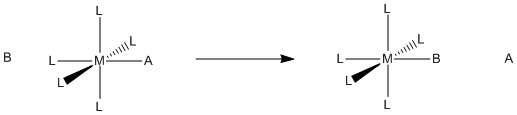
Figure LS1.1. Substitution of one ligand for another in a coordination complex.
Reactivity in Chemistry
Ligand Substitution in Coordination Complexes
LS1. Introduction to Substitution
Ligand substitution refers to the replacement of one ligand in a coordination complex with another ligand.
Figure LS1.1. Substitution of one ligand for another in a coordination complex.
Remember, a ligand in coordination chemistry is just a Lewis base that binds to a metal atom or ion. It does so by donating a lone pair (or other pair of electrons). Generallly, this donation is reversible. The donor can always take its electrons back. Typically, there may be some balance between the metal's need for more electrons and the donor's attraction for its own electrons; donor atoms are frequently more electronegative than the metal.
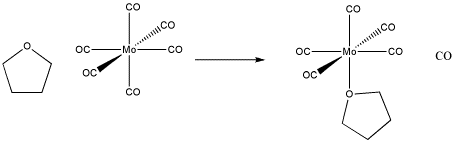
Figure LS1.2. An example of ligand substitution. THF replaces a carbon monoxide in this molybdenum complex.
Even though the reaction is pretty simple, it can occur in different ways.
That is, the elementary steps involved in the reaction can occur in different orders. The elementary reactions are the individual bond-making or bond-breaking events that lead to an overall change. Sometimes the order of steps is referred to as the mechanism or the mechanistic pathway.
You may have seen reaction mechanisms before. For example, carbonyl addition chemistry can involve lengthy mechanisms, in which a number of proton transfers and other bond-making and bond-breaking steps must occur to get from one state to another. Because ligand substitution is simpler than that, it is a good place to study mechanism in a little more depth, without getting overwhelmed by the details.
The sequence of steps in the mechanism influences how different factors will impact the reaction. For example, changing concentrations of different components in a reaction mixture can affect the time it takes for a reaction to finish.
These kinds of considerations have a dramatic impact on industrial processes such as pharmaceutical production. In that setting, chemical engineers need to make decisions about how much of each reactant must be admitted to a reaction mixture and how long they should be allowed to react together. If they allow the reaction to proceed for too, long, there may be �side-reactions� that start to occur, interfering with the quality of the product, and they will waste valuable time in the production pipeline. If they don�t allow it to react long enough, the reaction may not finish, and the product will be contaminated with leftover starting materials.
In this chapter, we will look at how this simple reaction can occur in different ways. We will see some different methods that are used to tell which way the reaction occurs (i.e. evidence of what is really happening). We will also look at some different factors that may influence whether the reaction is likely to occur one way or the other (i.e. reasons it is happening that way, or reasons we expect it will happen that way).
Problem LS1.1.
Some kind of substitution occurs in each of the following reactions: an atom or group replaces another. In each case, identify what is being replaced, and what replaces it.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Back to Ligand Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry