Reactivity in Chemistry
Ligand Substitution in Coordination Complexes
LS3. Evidence of Mechanism: Reaction Kinetics
How can you confirm through experiment that the reaction is happening in a particular way? There are lots of experiments people perform to work out how reactions happen. One of the methods used is chemical kinetics, in which the rate of a reaction is measured. By making changes in the reaction conditions and measuring the effect of the changes on the rate of reaction, we can infer what is going on at the molecular level.
Historically, the easiest way to measure the speed of a reaction is to measure the concentration over time. We can measure either the concentration of the reactants (the starting materials that are reacting) or the products (the compounds formed at the end of the reaction).
We sometimes write the rate of the reaction as:
Rate = d[product]/dt
Meaning, the rate is the change in concentration of product with change in time.
If you aren't very familiar with the idea of concentration, it really refers to how populated a solution is with a particular compound.

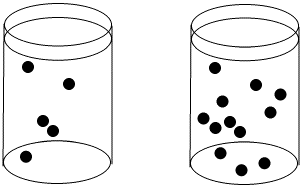
Figure LS3.1. The concentration of black dots is higher in the beaker on the right than in the beaker on the left.
Concentration could be measured in any units. Frequently, we are dealing with a solution, and we use units such as grams per liter or, much more commonly, moles per liter. The change in time is most often measured in seconds.
We could also write the rate of the reaction as:
Rate = -d[reactant]/dt
Meaning, the rate is the change in concentration of reactant with change in time. The minus sign just means that the reaction is getting consumed over time as it turns into product, so its concentration is decreasing.
Kinetic studies are important in understanding reactions. Not only are they important in industry, but they are also used to understand biological processes, especially enzyme-catalyzed reactions. They also play a role in environmental and atmospheric chemistry, as part of an effort to understand a variety of issues ranging from the fate of prescription pharmaceuticals in wastewater to the cascade of reactions involved in the ozone cycle.
Problem LS3.1.
Suppose the rate of the reaction between the black circles and the white circles depends only on the concentration of the black circles. That is, rate = k [black circle]. Compare the rate in each case to the rate of the reaction that would occur in the original beaker.
Problem LS3.2.
Suppose the rate of the reaction between the black circles and the white circles depends on both the concentrations of the black circles and the white circles. That is, rate = k [black circle][white circle]. Compare the rate in each case to the rate of the reaction that would occur in the original beaker.
Problem LS3.3.
Suppose the rate of a reaction in the beaker depends on the surface area of the solid at the bottom of the beaker. That is, rate = k x (surface area of white circles). Compare the rate in each case to the rate of the reaction that would occur in the original beaker.
Problem LS3.5.
Often in studying reaction kinetics, the changing concentration of a reactant or a product is plotted against time. In one method, many data points are collected very early in a reaction (when fewer than 5% of the material has reacted), and the slope of the resulting line is used to determine the "initial rate". Explain why this method might not work if the data points are plotted all the way until the reaction is finished.
Problem LS3.6.
Suppose the following plots were obtained before 5% conversion for the reaction:
A + B ---> C
What do you know about the rate law for the reaction?
Problem LS3.7.
Suppose the following plots were obtained before 5% conversion for the reaction:
A + B ---> C
a) What is a possible rate law for the reaction?
b) Two different rate laws could explain this data. What is the second possible rate law?
c) Propose an experiment to distinguish between these two possible rate laws.
Problem LS3.8.
Suppose the following data were obtained by monitoring the following reaction to completion:
A + B ---> C
a) How long does it take until the reaction is essentially finished, if the starting concentration of A is:
i) 1 mol/L?
ii) 2 mol/L?
iii) 4 mol/L?
b) What do you know about the rate law for the reaction? Explain.
Problem LS3.9.
Suppose the following data were obtained by monitoring the following reaction to completion:
A + B ---> C
Compare this graph to the one in the previous problem. What differences can you detect in the curves? Do you think this reaction has the same rate law as the previous one?
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Ligand Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry