Reactivity in Chemistry
Electrophilic Aromatic Substitution
AR10. Aryl Diazonium Coupling Reactions
We have seen how a nitro group can be converted to an amine via reduction with HCl and an active metal, such as tin or iron. The amine group can then be converted to an aryl diazonium group, Ar-NN+ X-, via treatment with sodium nitrite and acid. These diazonium ions undergo a range of reactions in which dinitrogen (N2) is displaced by an apparent nucleophile.
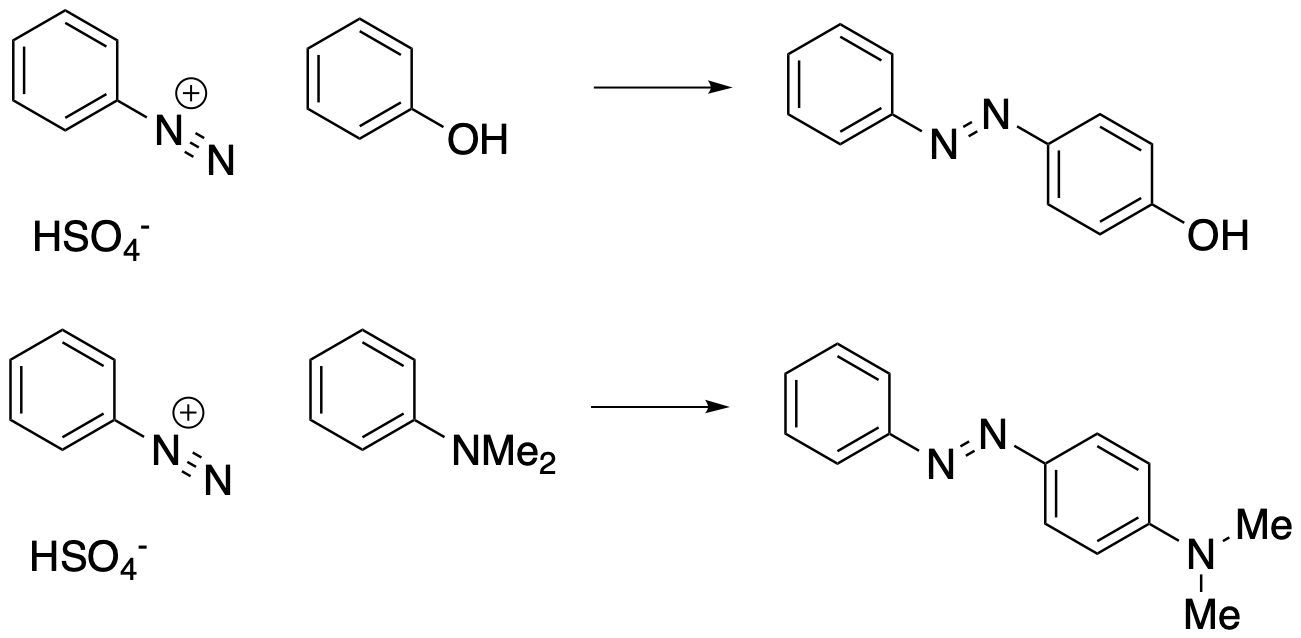
Aryldiazonium ions also undergo another kind of reaction in which the N2 group attaches to another benzene ring, forming a bridge between two benzenes. This is called a diazonium coupling reaction. In a diazonium coupling reaction, the diazo group acts as an electrophile. The positively charged nitrogen is electron-deficient and it attracts electrons from a nucleophile. The nucleophile doesn't donate to the positive nitrogen directly, however. Instead, the nucleophile donates to the end nitrogen or terminal nitrogen. When that happens, electrons from the pi bond are released; they become a lone pair on the middle nitrogen and the positive charge on that nitrogen goes away.

Figure AR10.1. Examples of diazonium coupling.
The nucleophile in a diazonium coupling is another benzene ring. From the nucleophile's point of view, this is just another electrophilic aromatic substitution. A pi bond is donated to the diazonium, forming an arenium ion intermediate. Deprotonation of the arenium ion leads to restoration of aromaticity.
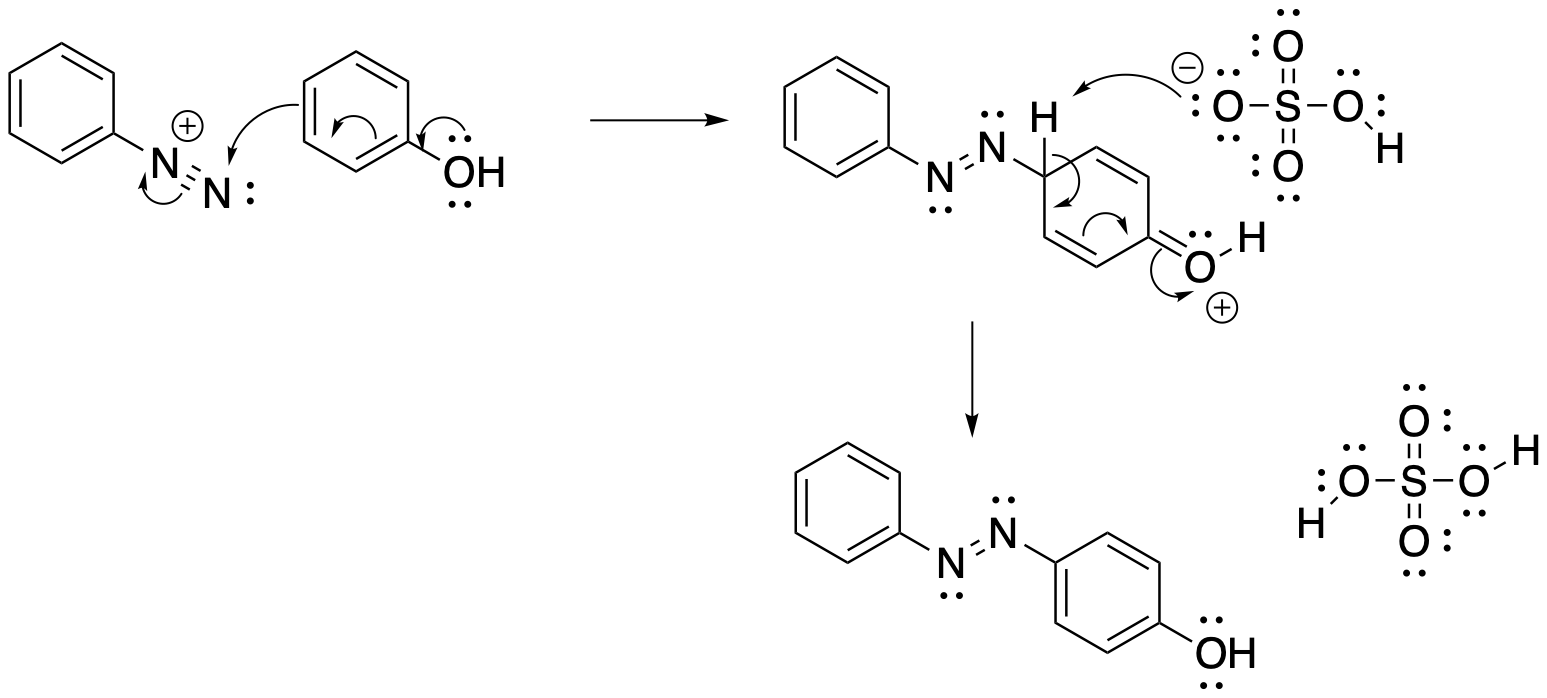
Figure AR10.2. Mechanism of diazonium coupling.
Problem AR10.1.
Draw a mechanism with intermediates and curved arrows for the coupling reaction of the PhN(CH3)2 with PhN2HSO4.
The diazonium coupling reaction leaves a N=N unit between two benzenes. This conjugated system is long enough that its UV-visible absorption moves into the visible range. That makes the product of a diazonium coupling reaction brightly coloured. As a result, these compounds are often used as pigments in paint or dyes in other situations. Pigments from this class of compounds are called "azo dyes".
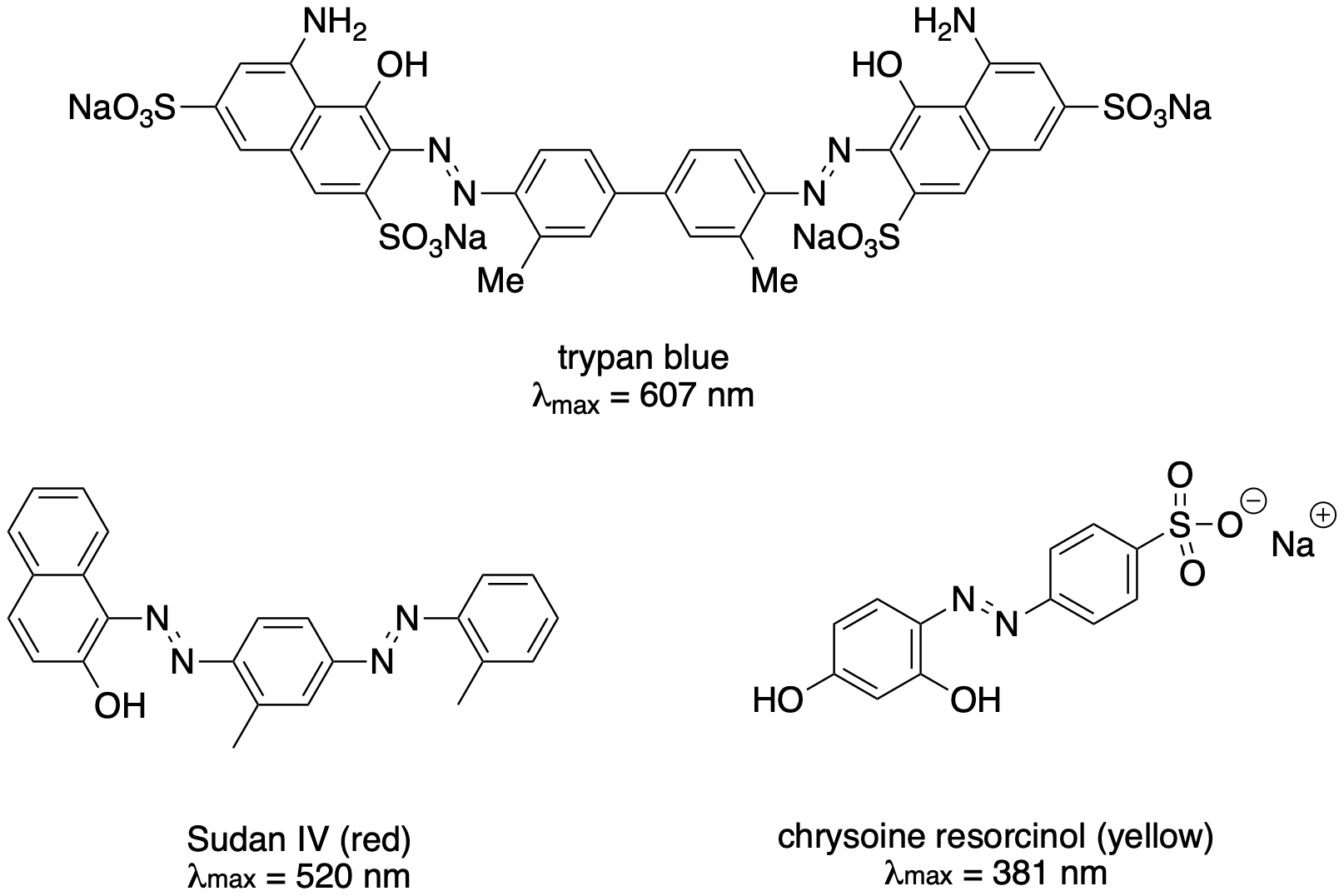
Figure AR10.3. Examples of azo dyes.
It is pretty common for the nucleophile in a diazonium coupling to contain oxygen or nitrogen groups such as alcohols, ethers, or amines. These pi donors make the nucleophile more strongly electron-donating, which helps the coupling reaction. Remember, these groups are activating in electrophilic aromatic substitution reactions.
Problem AR10.2.
Show the diazonium electrophile and the aromatic nucleophile that could be used to make each of the following pigments.
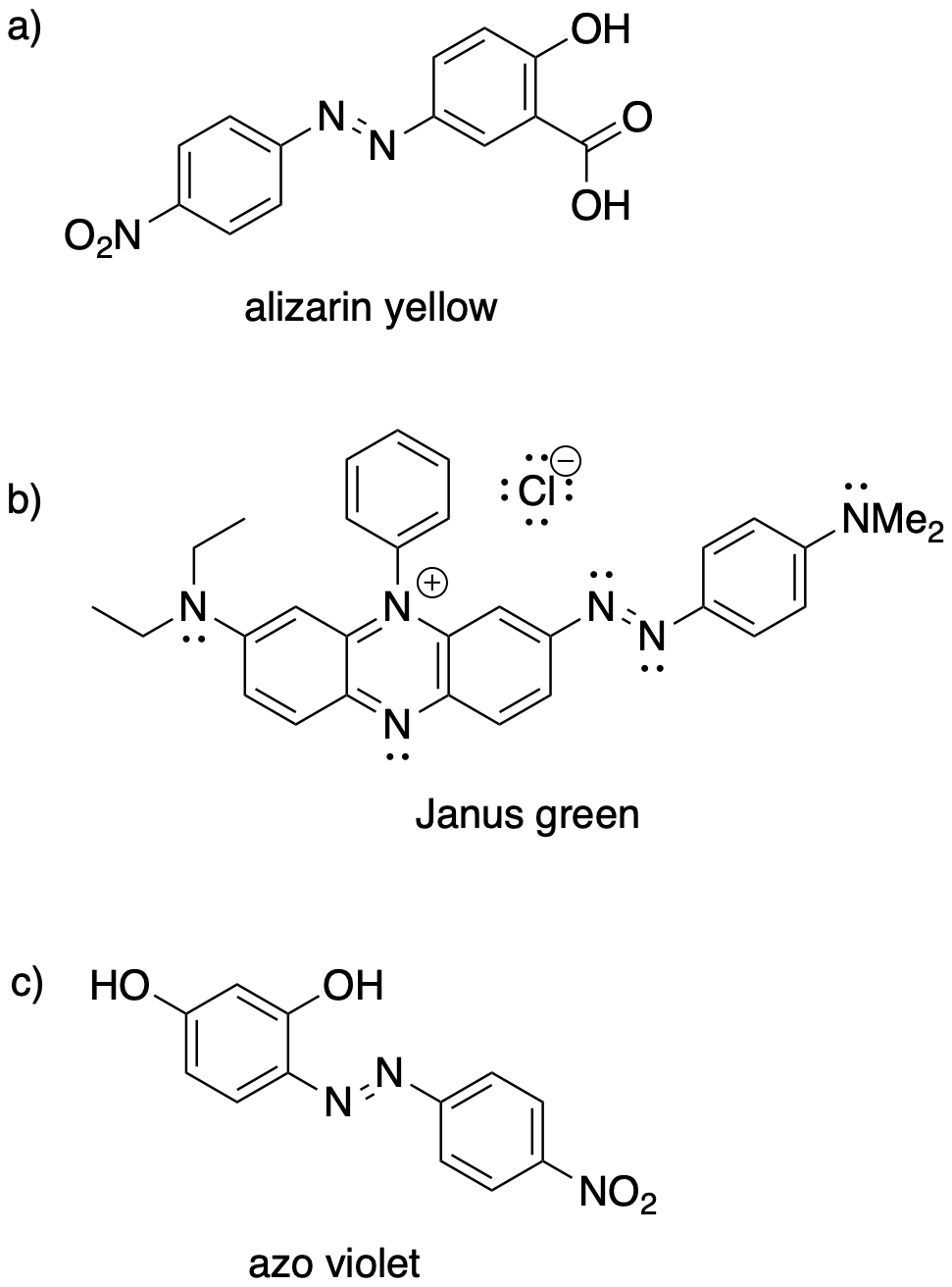
Problem AR10.3.
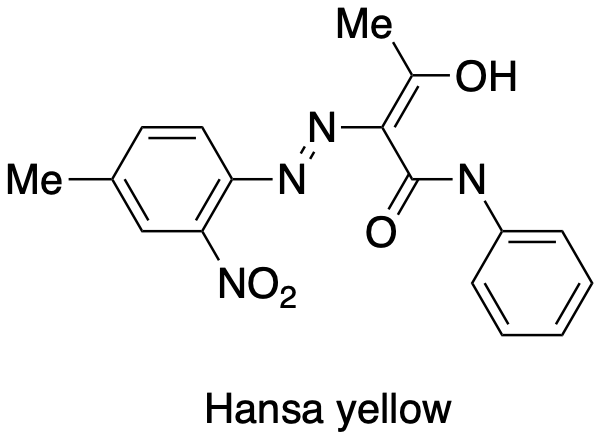
Hansa yellow is term to describe a family of pigments widely used in paints to deliver a bright yellow hue. Although it is an azo dye, formation of Hansa yellow does not use the usual aromatic nucleophile. Show the diazonium electrophile and the nucleophile that could be used to make this pigment.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: