Reactivity in Chemistry
Electrophilic Aromatic Substitution
AR3. Formation of the Electrophile
The mechanism of electrophilic aromatic substitution follows two elementary steps. First, donation of a pair of π electrons to the electrophile results in a loss of aromaticity and formation of a cation. Second, removal of a proton from that cation restores aromaticity.
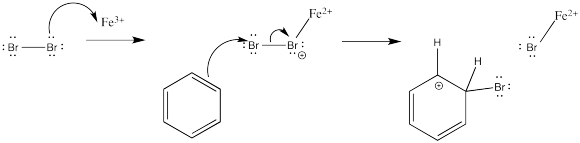
How does the electrophile form in the first place? The details of that part of the reaction vary from case to case. With the catalysed bromine reaction, the Lewis acid activates the halogen to render it more electrophilic. The activation may even go so far as to form a bromine cation, as suggested earlier. Otherwise, the positive charge on the bromine atom that ligates the Lewis acid can be nullified, indirectly, when the arene donates to the terminal bromine atom.

Figure AR3.1. Activation and reaction of a bromine electrophile.
The appearance of a bromide ion to deprotonate the cation simply results fom the equilibrium of the Lewis acid-base complex.

Figure AR3.2. Reversibility of a Lewis acid-base complex.
Problem AR3.1.
Show the mechanism for chlorination of benzene in the presence of ferric chloride.
The reactions of alkyl and acyl halides also involve Lewis acid catalysts; frequently, aluminum chloride (AlCl3) is employed. These two reactions are called Friedel-Crafts reactions after the French and American co-discoverers of the reaction. Typically, Friedel-Crafts reactions are believed to occur through initial formation of cationic electrophiles, which then react with aromatics in the same way as halogen electrophiles.
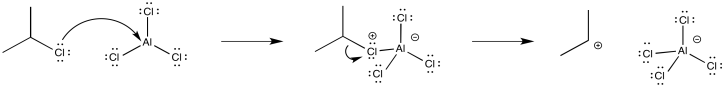
Figure AR3.3. Formation of an alkyl electrophile.
The alkyl cation is a potent electrophile. It is able to temporarily disrupt the aromaticity of the aromatic ring, forming an arenium ion.
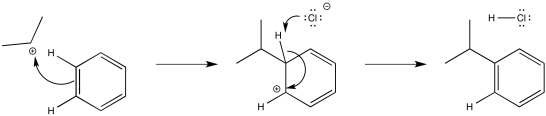
Figure AR3.4. Reaction of an alkyl electrophile with benzene.
The arenium ion intermediate is probably deprotonated by halide ion; some amount of these ions would be in equilibrium with the Lewis acid-base adduct.
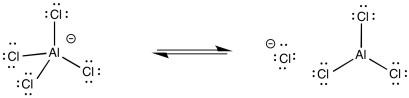
Figure AR3.5. Reversibility of a Lewis acid-base complex.
Because Friedel-Crafts alkylations occur via alkyl cations, the reactions of primary alkyl halides are generally pretty slow. Those cations just aren't stable enough to form. Although it sometimes seems like formation of a stable intermediate would slow things down, because it would not be motivasted to react further, usually the reverse is true. Because an intermediate is at high energy, it is inherently difficult to form in the first place. This difficulty acts as a blockade on the reaction, so that it doesn't proceed very easily.
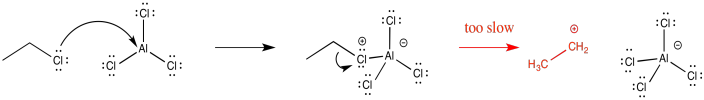
Figure AR3.6. An unstable ethyl cation.
Forming the intermediate more easily, therefore, allows the reaction to proceed more quickly. For example, the tert-butyl cation is relatively stable, so that intermediate is formed relatively easily. Alkylation of aromatics rings with tertiary alkyl halides is especially easy to accomplish.
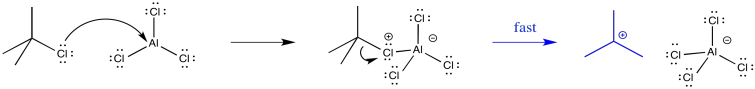
Figure AR3.7. Rearrangement of a butyl cation.
In some cases, formation of a cation probably does not happen at all. Instead, the activated Lewis acid-base complex acts as the electrophile directly. This pathway seems to occur with methyl electrophiles. However, there are also indications that primary alkyl halides undergo this mechanism in parallel with the cationic mechanism. That means there are two competing reaction mechanisms, possibly leading to mixtures of products
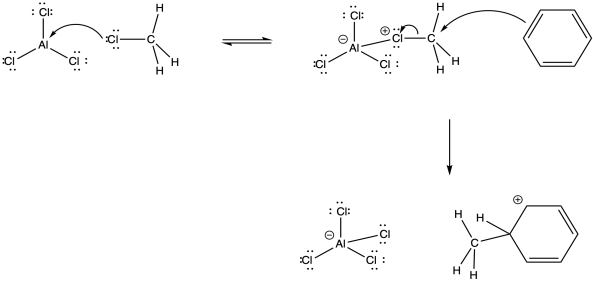
Figure AR3.8. The failure to form a CH3+cation.
It is also worth noting that in some cases, such as with longer alkyl chains, multiple products may result via rearrangements. These observations provide additional evidence for the cationic nature of the intermediates as well as competing pathways.
Problem AR3.2.
Show the mechanism for the Friedel-Crafts alkylation of benzene with 2-chloropropane in the presence of aluminum chloride.
Problem AR3.3.
Why is the Friedel-Crafts reaction of 1-chloropropane so much slower than the reaction of 2-chloropropane? Explain using a mechanism and intermediates.
Problem AR3.4.
Show why Friedel-Crafts alkylation of benzene with 2-chloropentane results in the formation of two different products.
Problem AR3.5.
Show the mechanism for the Friedel-Crafts acylation of benzene with ethanoyl chloride (acetyl chloride) in the presence of aluminum chloride.
Problem AR3.6.
Show why the Friedel-Crafts acylation of benzene with pentanoyl chloride results in only one product, with no rearrangement.
Nitration and sulfonation reactions differ from the other substitutions that we have seen because they do not utilize Lewis acid catalysis. These reactions depend on equilibria that occur in strongly acidic media. When nitric acid is dissolved in sulfuric acid, there is spectroscopic evidence than NO2+ forms, providing an electrophile. That electrophile adds readily to the aromatic ring. The formation of NO2+ results from dehydration of nitric acid, the loss of water when exposed to strongly acidic conditions.
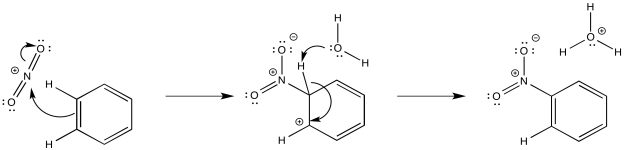
Figure AR3.9. Reaction with a nitronium ion.
Similarly, when sulfuric acid is concentrated by boiling off residual water, sulfur trioxide results. The dehydration of a molecule of H2SO4 leads to the formation of SO3; it's a reaction that is similar to the dehydration of nitric acid. Because it is formed in concentrated sulfuric acid, the sulfur trioxide is probably in a protonated state. The sulfur trioxide easily forms SO3H+. That would make it an even stronger electrophile than plain SO3.
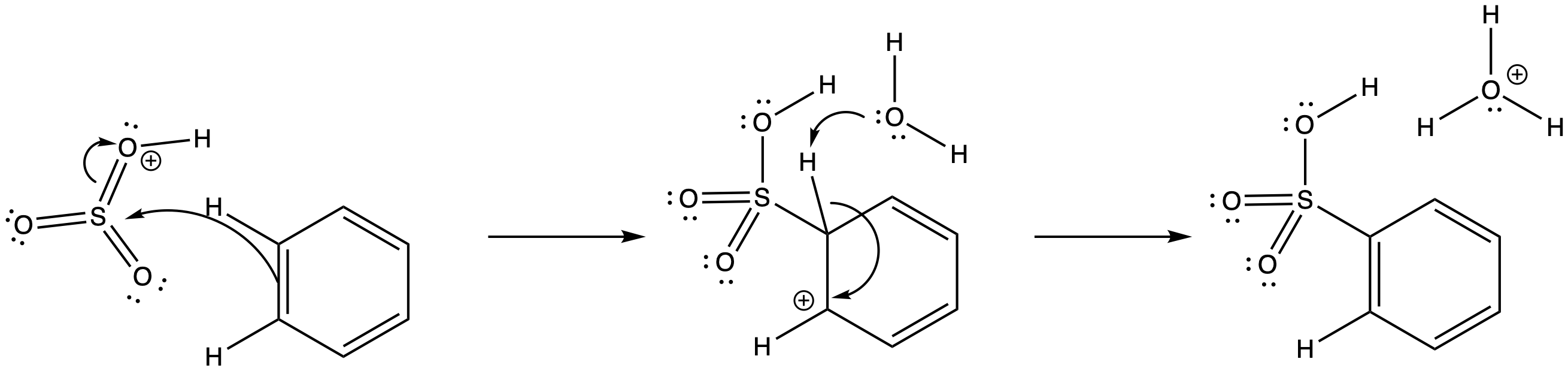
Figure AR3.10. Reaction with sulfur trioxide.
Problem AR3.7.
Provide a mechanism for the formation of NO2+ from nitric and sulfuric acid.
Problem AR3.8.
Provide a mechanism for the formation of SO3H+ from sulfuric acid.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: