Reactivity in Chemistry
Reduction Potentials of Metal Ions in Biology
MB2. The Effect of Charge (Local Effects)
Electron transfer reactions result in changes in charge. Issues of charge stability are therefore crucial in determining whether or not a reaction is favoured. If the donation of an electron will result in increasing negative charge on a complex, then donation may be inhibited. That's because the buildup of charge is energetically unfavourable. On the other hand, if donation of an electron would result in decrease of charge on the complex, then donation may occur more easily.
Remember: this is just one of several factors that could influence the behaviour of a coordination complex. Strict rules do not apply here.
Problem MB2.1.
Calculate the overall charges on the following metal complexes, given the indicated oxidation state on the metal.
a) AgI(NH3)2 b) FeII(H2O)6 c) CuII(NH3)4 d) AgICl2
e) CoIII(NH3)4Cl2 f) HgIII4 g) FeII(CN)6 h) CoII(SCN)4
i) CrVIO4 j) CoIII(NO2)3(NH3)3 k) CoIII(NH2CH2CH2NH2)2Br2 l) ReI(CH3)(CO)5
Problem MB2.2.
Given the overall charges on the following coordination complexes, calculate the oxidation state of the metal.
a) [CuCl4]2- b) [Pt(NH3)4]2+ c) [Au(CN)2]- d) [CrF4]2+
e) [PtCl6]2- f) [FeCl(CN)5]3- g) [Pt(NH2CH2CH2NH2)2Cl2]2+ h) [Cu(CF3)4]-
i) [Ni(C2O4)2(H2O)2]2- j) [CoCl(NO2)(NH3)4]+ k) [Co(NH3)4SO4]- l) [Ag(S2O3)2]3-
Problem MB2.3.
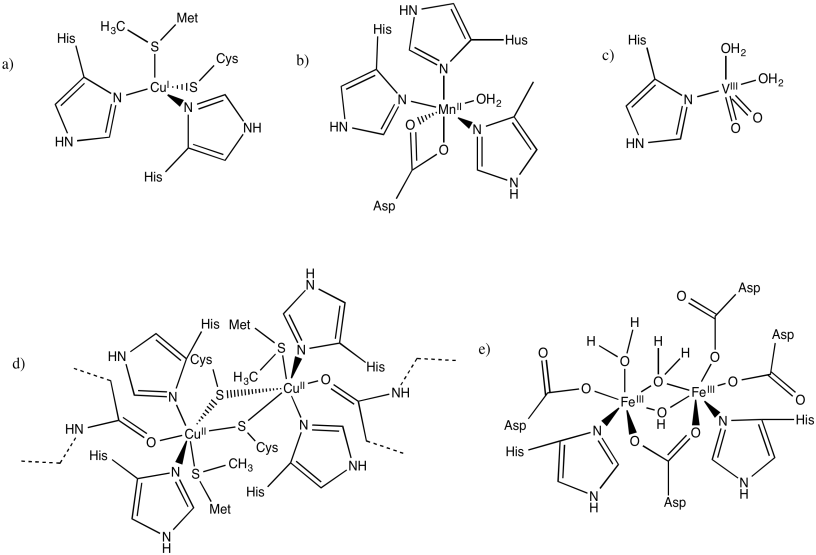
Indicate the charges on the central complex in the following biological sites. (The entire protein would have a charge, depending on amino acid residues; we are just interested in the local charge that we can see here.)

Upon reduction, the complex will become more negatively charged, because it is accepting an electron. Alternatively, it may become less positively charged. In any case, the electron will be more easily accepted into a complex with a lower negative charge to begin with. Build up of charge costs energy.
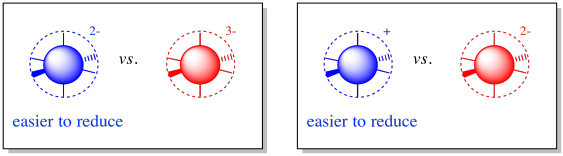
Figure MB2.1. The charge on a complex influences its reduction potential.
A good example of this charge effect can be seen among iron-sulfur clusters. Iron-sulfur clusters frequently participate in electron transfer pathways in biology. The range of reduction potentials among these proteins is quite wide, ranging from -700 to +400 mV.
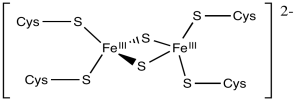
Figure MB2.2. A typical FeS cluster.
Typically these clusters contain multiple iron and sulfur atoms and are held in place by cysteine residues from a surrounding protein. The sulfur atoms are present as sulfide anions (S2-); there are usually two or four of them in the cluster. The cysteine residues are typically deprotonated, so they act as thiolate anions (RS-). The number of cysteine residues attached to the cluster varies. Usually there is a sufficient number to give the iron a tetrahedral geometry. The iron atoms can be present as Fe(II) or Fe(III), so charges can vary. There might be two, three or four iron ions in the cluster. The most common combinations are two iron and two sulfur atoms or else four iron and four sulfur atoms.
Rieske iron centres are "high-potential iron proteins", meaning they have especially positive reduction potentials. They have histidines in place of cysteines in some positions. As a result, the central coordination complex is more positively charged, or at least less negatively charged. Because the central complex is less negatively charged, it can more easily accept an electron than other iron sulfur proteins.
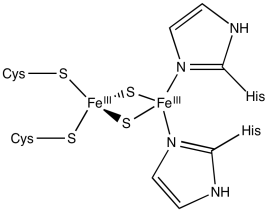
Figure MB2.3. A Rieske FeS cluster or high-potential FeS cluster.
Problem MB2.4.
Calculate the charges on the following iron-sulfur complexes.
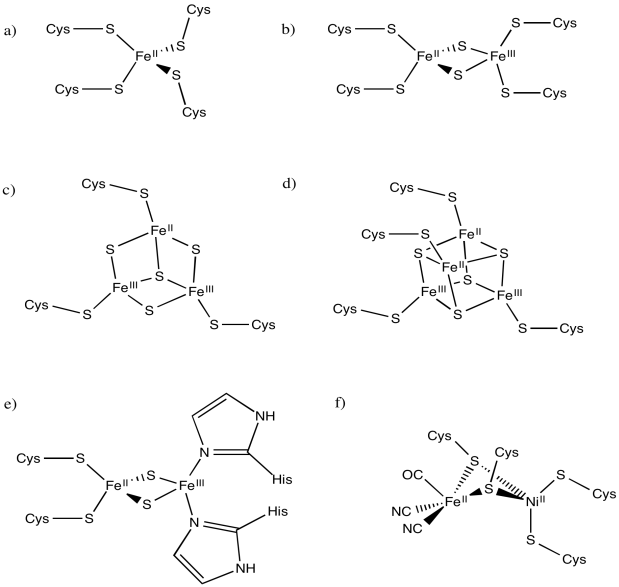
Problem MB2.5.
The following iron complexes have multiple possible oxidation states. What is the range of possible charges on each complex, assuming each iron atom could be Fe(II) or Fe(III)?
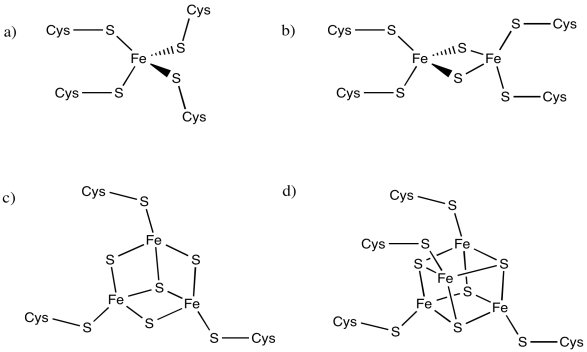
Problem MB2.7.
In order to build a better understanding of the properties of metalloproteins, the Pecoraro lab at University of Michigan has studied the binding of synthetic peptides to copper (J. Am. Chem. Soc. 2013, 135, 18096-18107.) Some of the synthetic peptides are shown below, along with the reduction potentials for the bound copper species:
(peptide)3CuII + e- → (peptide)3CuI
| Peptide | Sequence | E° |
| A | Ac-G WKALEEK LKALEE LKALEEE HKALKEK G-NH2 | 504 mV |
| B | Ac-G WKALEEK LKALEE LKALEEE HKALQEK G-NH2 | 474 mV |
| C | Ac-G WKALEEK LKALEE LKALEEE HKALEEK G-NH2 | 440 mV |
a) The copper binds to the histidine. Draw the structure of the binding site.
b) Calculate the local charge at the binding site when bound to (i) CuI and (ii) CuII.
c) If the overall charge on the peptide complex A3CuII is zero, what are the charges on (i) B3CuII and (ii) C3CuII?
d) Suggest a reason for the change in the reduction potentials between the three complexes.
e) By comparison, the reduction potential of aqueous CuII is 159 mV. Suggest a reason for the difference bteween the reduction potential of aqeous copper and the peptide complexes.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: