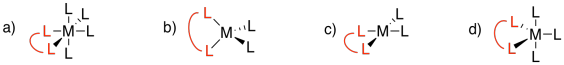CC4. Chelation
Monodentate ligands bind through only one donor atom. Monodentate means "one-toothed". The halides, phosphines, ammonia and amines seen previously are monodentate ligands.
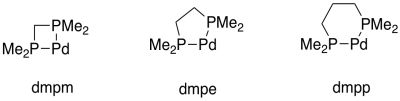
Bidentate ligands bind through two donor sites. Bidentate means "two-toothed". An example of a bidentate ligand is bis(dimethylphosphino)propane. It can bind to a metal via two donor atoms at once: it uses one lone pair on each phosphorus atom.
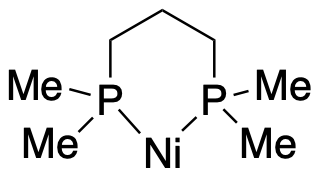
Figure CC4.1. An example of a bidentate ligand coordinated to a metal.
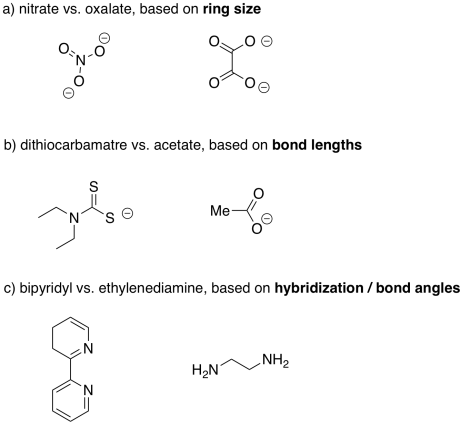
More examples of bidentate ligands are shown below. They all have at least two different atoms with lone pairs. In some cases, there are additional atoms with lone pairs, but only two of them are able to face the metal at one time. Oxalate and glycinate would act as bidentate donors, donating up to two sets of lone pairs at the same time.
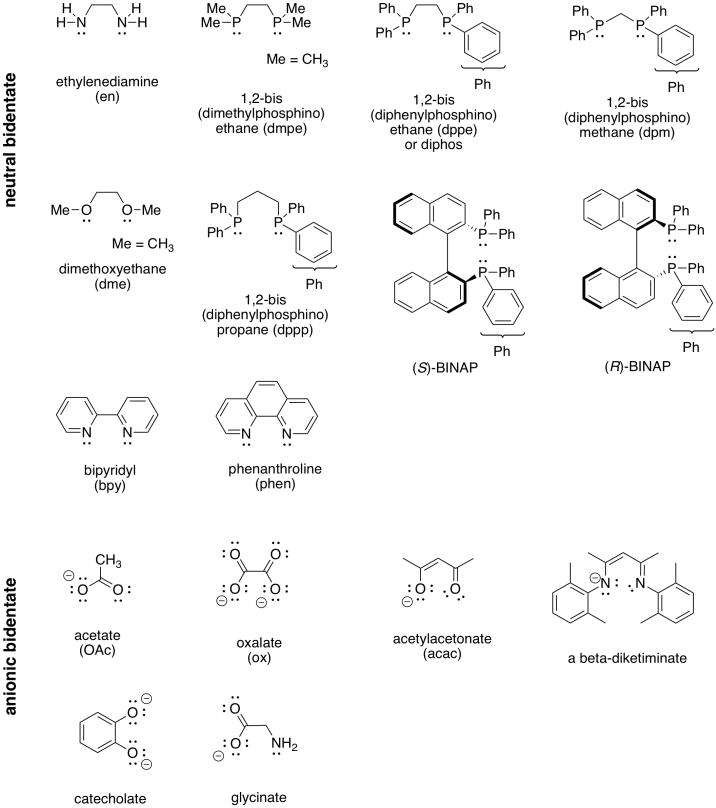
Table CC4.1. Some common bidentate ligands
Bidentate binding allows a ligand to bind more tightly. Tridentate ligands, which bind through three donors, can bind even more tightly, and so on. This phenomenon is generally called the "chelate effect". This term comes from the Greek chelos, meaning "crab". A crab does not have any teeth at all, but it does have two claws for tightly holding onto something.for a couple of reasons. A very simple analogy is that, if you are holding something with two hands rather than one, you are not as likely to drop it.
-
Multidentate ligands bind more tightly because of the chelate effect
The chemical reasons for the chelate effect involve relative enthalpy and entropy changes upon binding a multidentate ligand. In terms of enthalpy, in order to completely remove a bidentate ligand, two coordinate bonds must be broken. That costs more energy than breaking one coordinate bond for a monodentate ligand.
In terms of entropy, which deals with the distribution of energy within a system, it is generally thought that bringing two molecules together (a bidentate ligand and a metal complex) costs less than bringing three molecules together (two monodentate ligands and a metal complex). That's because individual molecules are free to move around, tumble and vibrate independently. Once they come together, they have to do all these things together. Since these different types of motion represent different ways of distributing energy, if the system becomes more restricted, energy can't be distributed in as many states.
-
Energy is lowered even more by two bonding interactions
-
Compared to two separate donors, bidentate donation is entropically favoured
Problem CC4.1.
Draw metal complexes using the ligands below, binding to Ni(2+) in a bidentate mode.
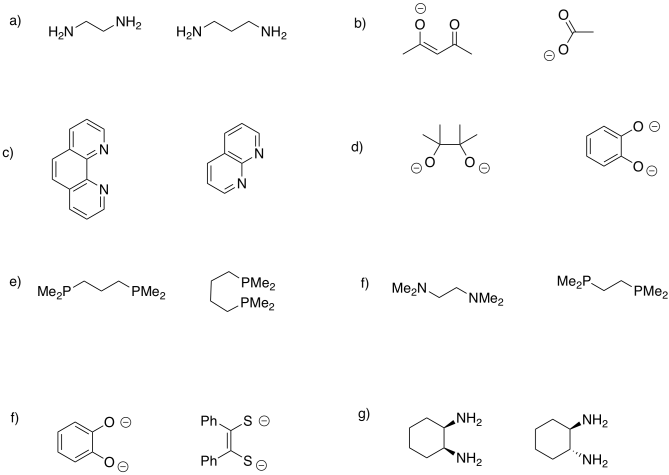
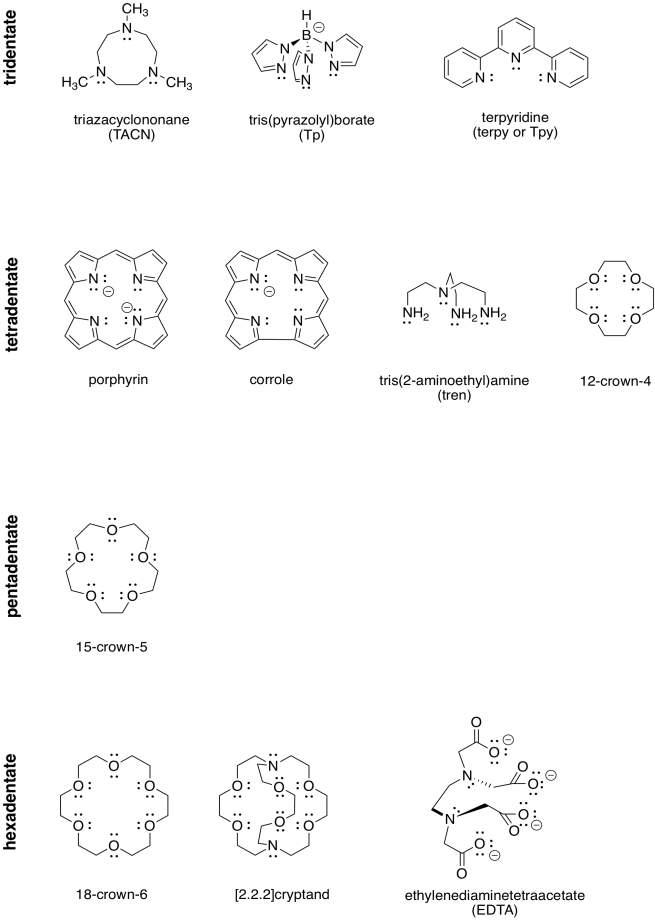
A ligand could be monodentate, meaning it binds through a lone pair on a single atom. It could be bidentate, meaning it binds through lone pairs on two different atoms. It could even be tridentate, with three atoms bearing their own lones pairs, tetradentate, and so on.
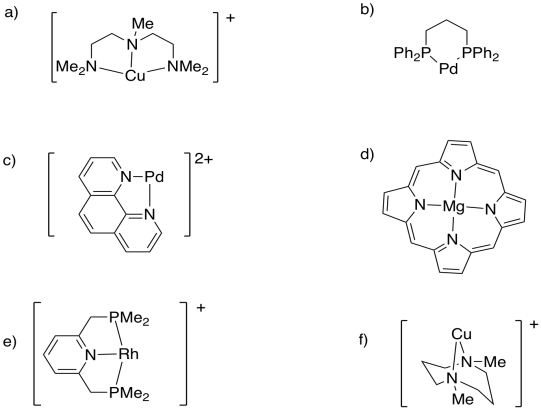
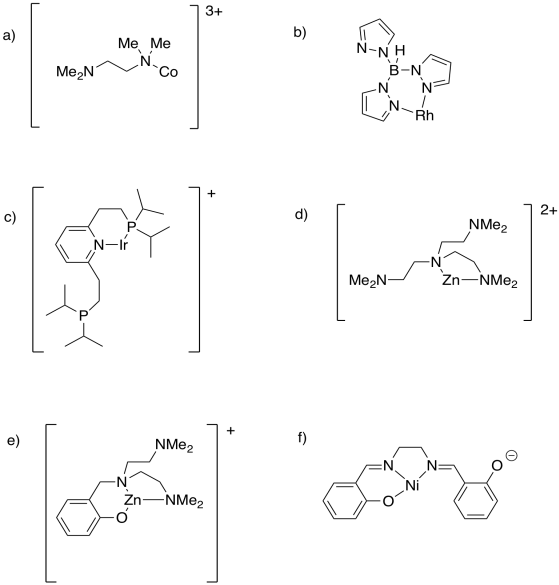
Table CC4.2. Examples of polydentate ligands.
There is a symbol for denticity, κ (it's a Greek letter, pronounced "kappa"), which simply describes how many atoms are bound to the metal. For example, in ethylenediamine or 1,2-diaminoethane, NH2CH2CH2NH2, the two nitrogen atoms can be bound to the metal at the same time, although none of the other atoms in between would be directly attached to the metal. This donor is capable of binding in a κ2 mode. However, if for some reason one of the nitrogen atoms lets go of the metal so that the ethylenediamine is hanging on by only one nitrogen, we would say that the ligand is binding in κ1 mode.