CC3. Electron Counting in Transition Metal Complexes
Electron counting is always important in chemistry, especially when reactions are occurring. Because reactions involve the transfer of electrons from one atom to another during the making and breaking of chemical bonds, we need to keep track of where the electrons are going.
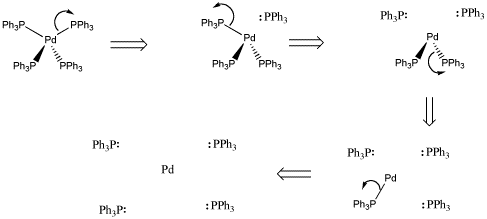
Counting electrons on a transition metal in a coordination compound can be a little tricky. Instead of an eight-electron rule or octet, transition metals obey an eighteen electron rule. The easiest way to count electrons is to take the complex apart and count the electrons in pieces. First, we give the donated electrons back to the individual ligands.
-
Deconstruct the complex: give lone pairs back to ligands
Figure CC3.1. Giving each pair of donor electrons back to the ligands in a coordination complex.
Once the complex has been deconstructed, we count a pair of electrons for each ligand, since they are each donating a pair to the metal in the complex. Also, we must count the valence electrons that the metal brings itself. The total is the electron count in the metal in the complex. It often equals eighteen.
-
Count one pair of electrons per ligand
-
Count the valence electrons on the metal
Figure CC3.2. Counting electrons on the ligands and the metal.
There is a wrinkle in this process if charges are involved. For example, if the free ligands are not neutral, but charged, you need to adjust the electron count on the metal. The metal may be an ion, not an atom, so the electron count will be lower. If donor atoms have formal charges, adjust the charge on the metal atom or ion to balance the overall charge on the complex. In many cases, the overall charge is zero, so the metal charge is just the sum of charges on ligands.
If you don't remember anything about formal charges, review here.
-
Check for formal charges on free ligand donor atoms
-
Adjust charge on metal so that the overall charge of (ligands + metal) equals the charge on the complex
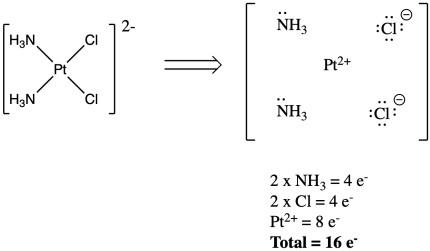
For example, in cis-platin, two of the ligands are chlorides, which have negative charges. Since cis-platin is neutral overall, the platinum must have a two plus charge. As a result, it does not have ten electrons. It only has eight. The total electron count on the metal in the complex is sixteen.
Figure CC3.3. Counting electrons on the anionic ligands and the metal ion.
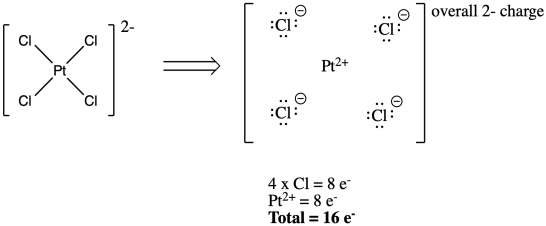
A further wrinkle occurs if there is an overall charge on the complex. In main group, covalent compounds, we always adjust the electron count to reflect an overall charge on the structure. We don't have to do that here, because we already make the adjustment when we decide the "oxidation state" or charge on the metal.
Figure CC3.4. Counting electrons on the ligands and the metal ion in an overall anionic complex.
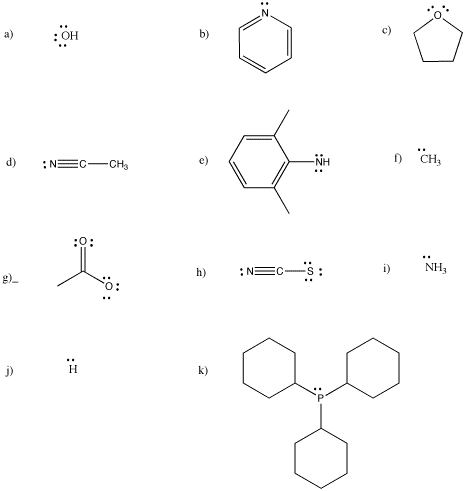
Anionic ligands are often prepared by deprotonating a neutral molecule. Some common bases used in synthetic chemistry are shown below. A range of base strengths are needed to ensure complete removal of proton from a variety of reactants. On the other hand, very large pKa differences between the acid reactant and the conjugate acid produced in the reaction, can cause highly exothermic reactions and lead to fires.
Table CC3.1. Some bases commonly used in synthetic chemistry.
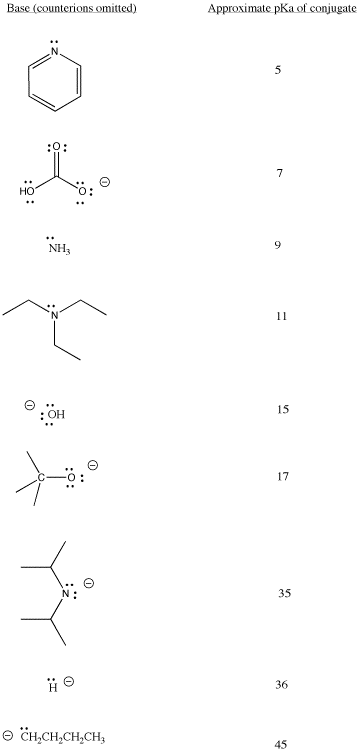
Table CC3.1. Some bases commonly used in synthetic chemistry.
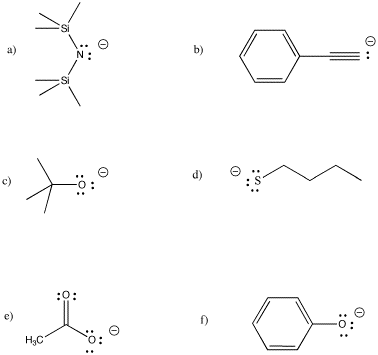
Problem CC3.1.
Which of the following ligands are anionic, and which ones are neutral?
Problem CC3.2.
For the following ligands, show the corresponding neutral (the conjugate acid). Select an appropriate base to deprotonate it, and justify your choice.