CC6. Hapticity
When a lone pair is bonded to a transition metal, then the atom with the lone pair is forming a bond to the metal. When a pi bond is coordinated, which atom is bonded to the metal? Both of them. Three atoms are involved in this bonding situation, instead of just two. Both carbons that form the original pi bond are now donating that pi bond to the metal.
When ligands are bound to a metal via a conjugated pi-system, describing the mode of binding might seem even trickier. If there are two double bonds in a row, then all four of the atoms that form those two pi bonds are donating to the metal. Furthermore, because the bond is conjugated, we can think of this as one long pi bond. The bond from the pi bond to the metal involves all four donor atoms, plus the metal atom.
Contrast that situation with two separate pi bonds that are not conjugated. If a ligand contains two separate pi bonds, it is a bidentate donor. Bidentate ligands bind through two donor sites. We think of a ligand like 1,2-ethanediamine as binding through the lone pairs on both nitrogen atoms. We would think of 1,5-hexadiene as binding through the pi bond at either end of the chain. However, 1,3-butadiene is a little different, because of the participation of all four carbons in bonding to the metal through one conjugated bond.
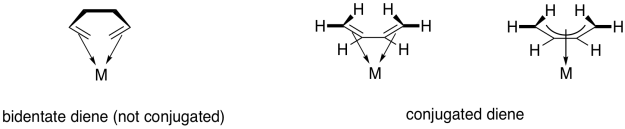
Figure CC6.1. An example of a tetrahaptic donor (right).
The term used to describe the participation of multiple atoms simultaneously during pi coordination is hapticity. A regular alkene, like ethene or propene, is a dihaptic donor; two carbons participate in donation of one bond to the metal. A conjugated alkene, like 1,3-butadiene, is a tetrahaptic donor. Four carbons participate in donation of a conjugated pi bond to the metal. Of course, this conjugated diene can donate four electrons at once, forming something a little like a double bond to the metal.
- "Hapticity" refers to the number of ligand atoms in a row that bond to the metal atom or ion.
- Hapticity is associated with ligands that have conjugated π systems.
- Just as with denticity, greater hapticity means greater strength of binding.
Table CC6.1. Some common multihaptic ligands.
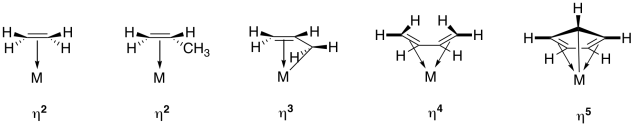
In the drawings above, the symbols, η2 or η3, etc. (read "eta-two" or "eta-three") refer to the hapticity of the ligand. An η2 ligand is dihaptic, with two atoms sharing in the donation from the pi system; an η3 ligand is trihaptic, with three atoms sharing in the donation from the conjugated pi system.
Problem CC6.1.
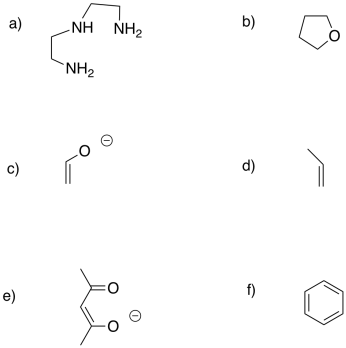
The following alkenes form complexes with silver. Describe their probable mode of binding as η2, etc.:
a) CH2CHCHCH2
b) CH2CHCH2CHCH2
c) CH2CHCH2CH2CHCH2
d) CH2CHCHCHCHCH2
Problem CC6.2.
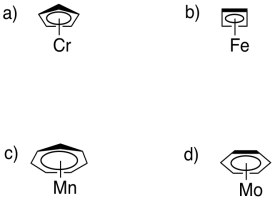
Cyclic, conjugated systems make good ligands for transition metals. In each of the following cases,
i) describe the hapticity.
ii) indicate the number of electrons donated to the metal.
iii) indicate the charge on the ligand.
Problem CC6.3.
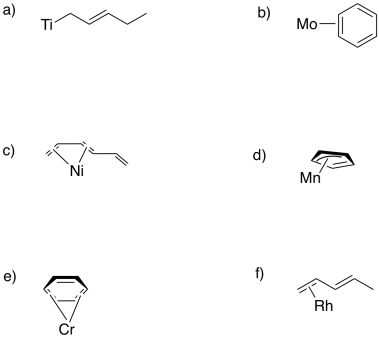
Sometimes, conjugated ligands might "slip", donating fewer than the maximum number of electrons to the metal. In the following cases, indicate:
i) the hapticity shown in the picture.
ii) the maximum hapticity possible with the ligand.
Problem CC6.4.
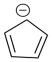
One of the most common multidentate ligands is the cyclopentadienyl anion, often abbreviated Cp.
a) CpH is easily deprotonated to form Cp-. Explain why.
b) How many electrons does Cp donate to a metal?
c) The archetypal Cp complex is ferrocene, Cp2Fe, the structure of which was determined by Geoff Wilkinson, in work that led to him being awarded the Nobel Prize in 1973. Draw the structure of ferrocene.
d) Count the electrons on the iron in ferrocene.
Problem CC6.5.