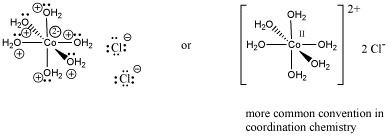Part CC1. Introduction
NOTE: A general introduction to coordination compounds is given in the context of Lewis acids and bases on another page.
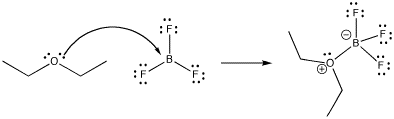
Much of reactivity can be understood in Lewis acidity terms. Perhaps the simplest examples of reactions are the formation of Lewis acid-base complexes. In a Lewis acid-base complex, a Lewis base has simply shared a pair of electrons with a Lewis acid, forming a new bond.
Figure CC1.1. Formation of a Lewis acid-base complex, boron trifluoride etherate.
Frequently, metal atoms or ions act as Lewis acids. They can often accept electrons from a number of different Lewis bases at once, forming "complexes" or "complex ions" ("complex" meaning they are formed from individual parts that connect together). These Lewis bases (also called "ligands") are said to be "coordinated" to the metal, meaning they are stuck to the metal via the electron pair that they share with it.
Figure CC1.2. Coordination of ammonia to complete the formation of cis-platin, an important antitumour drug.
- A coordination complex is made of one or more Lewis bases bonded to a Lewis acid.
- The Lewis acid could be a metal atom or metal ion.
- The Lewis base is normally called a "ligand".
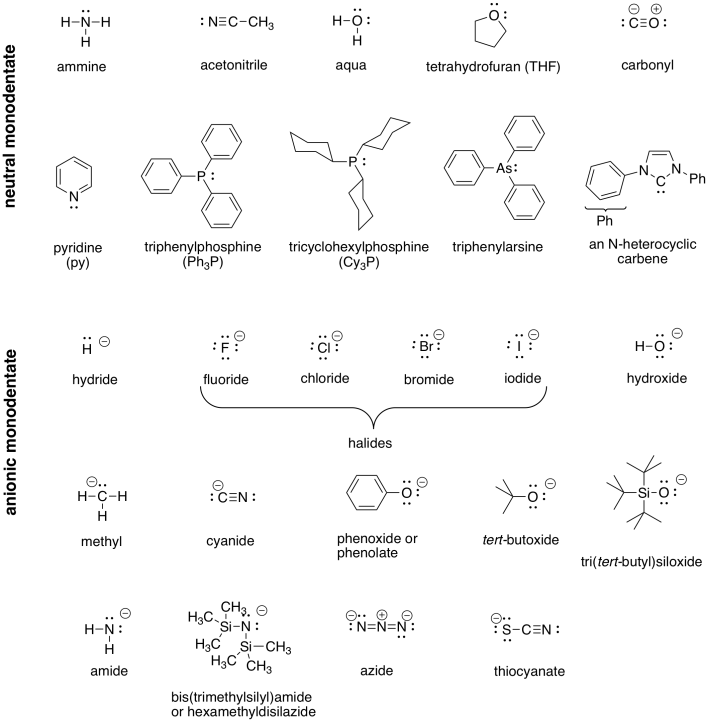
A number of examples of ligands (compounds with lone pairs that can bind to metals) are shown in the following table.
Table CC1.1. Some Common Ligands.
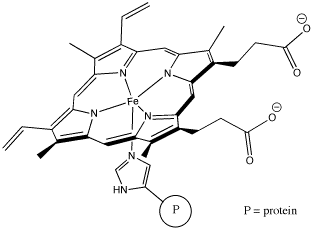
"Coordination complexes" play important roles in biology as well as economically important processes. Probably the most familiar coordination complex in biology is hemoglobin. It can coordinate with an additional dioxygen molecule and carry the oxygen through the bloodstream, delivering oxygen to tissues much more efficiently.
Figure CC1.3. Hemoglobin, a biologically important coordination complex.
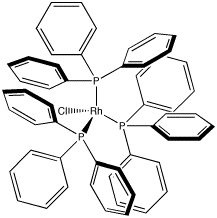
A very common coordination complex in industrial use is Wilkinson's catalyst, (PPh3)3RhCl. Wilkinson's catalyst is used to make a number of transformations more efficient; most notably, it is used in hydrogenation reactions. Chemical transformations of this sort are commonly used in making pharmaceuticals and other high-demand materials.
Figure CC1.4. Wilkinson's Catalyst.
Because coordination compounds can sometimes be anions or cations, there is a convention used to tell the reader which part of the formula is connected together, and which part is the counterion(s). The part listed in square brackets consists of ligands bonding to a central metal; the part outside the brackets is the counterion(s). For example, [(H2O)6Co]Cl2 consists of a Co2+ ion bound to six waters. Two separate chloride anions are found nearby.
Figure CC1.3. Structural drawings for the formula [(H2O)6Co]Cl2.
Problem CC1.1.
Draw structures for the following coordination compounds.
a) K2[PtCl6] b) K3[Fe(CN)6] c) [(NH3)4CoCl2]Cl (two isomers) d) [(NH3)3CoCl3] (two isomers) e)[(NH3)2Ag]PF6 (it's Ag+; that's a single PF6- counterion)
f) Na[HgCl3] g) Cd(NH3)4Cl2 (two isomers) h) Li2[CoCl4] i) K[Rh(CO)2I2] j) k) [Cu(NH3)4](SO4)2 l) Na2[Ni(CN)4]
m) [Fe(OH2)6])NO3)2 n) K3[Fe(SCN)6] o) K2[Zn(OH)4] p) Pt(NH3)2Cl2 (two isomers) q) [Ru(NH3)5Cl]Cl2 r) Li[Sn(OH)3]
s) K4[Mn(CN)6] t) Ni(CO)4 u) [Co(NH3)5NO2](NO2)2 (two isomers based on how the ligand connects to the metal)
v) [Co(NH3)5SCN]Cl2 (two isomers possible)
w) [VO]SO4 (challenge: why might you draw the V-O bond differently than the other metal-ligand bonds so far?)
x) [VO2]PF6 y) Na2[HgS2] z) K[MnO4]
Problem CC1.2.
Identify the geometries of each of the complexes in Problem CC1.1.
Note:
Some of the complexes in Problem CC1.1. have interesting stories. For example, problem (a) was reported many years ago by Alexander Shilov to be capable of breaking C-H bonds. This reaction has the potential to convert methane, routinely burned off in petroleum fields because it is a safety hazard, into useful products. All of that methane is currently being converted directly into greenhouse gases, and that's a real problem. (Astronauts have reported that, at night on Earth, the petroleum fields of the Middle East shine brighter than major cities.) This reaction has recently received intensive study in the lab of John Bercaw at Caltech.
Problem (i) is an economically important catalyst, used in the production of acetic acid in the Monsanto Process, originally developed by BASF but improved upon by Monsanto. These days, it is being supplanted by the same complex with iridium instead of rhodium; that complex drives the more environmentally-friendly Cativa Process. The new process was developed by BP. Acetic acid is a sort of heavy-duty worker molecule used in the production of paints, plastics, pharmaceuticals and other commodities.
Problem (p) is a member of a class of potent anti-cancer drugs that are particularly effective against testicular and ovarian tumours. Exactly how they worked was the subject of study for many years, and was finally deduced by Steve Lippard (MIT) and Amy Rosenzweig (Northwestern).
Problem (t) is used in the Mond Process for the purification of nickel ores. We use nickel principally in making steel, but also in other alloys.
Vanadyl salts such as (w) are sometimes found in minerals such as cavansite, giving them an intense blue colour.