CC13. Multiple Bonds in Coordination Complexes
Metal-Ligand Multiple Bonds
For the most part, we have looked at donor atoms that provide one pair of electrons to a metal. In chelation, two donor atoms on the same ligand can provide a total of four electrons to the metal. In addition, some ligands can form double (or triple) bonds to a metal, providing four or even six electrons from one donor atom.
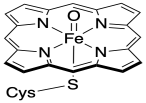
Oxides may be the most common multiply-bonded ligand. In biology, attention has turned to the role of iron and copper oxides as active intermediates in a variety of enzymes that use molecular oxygen to oxidize substrates. The most notable example is cytochrome P450, a ubiquitous class of oxidizing agents found in most organisms. In humans, cytochrome P450 is found in a variety of tissues, incorporating oxygen atoms into small molecules for a plethora of reasons. One interesting use of cytochrome P450 is as a detoxifying agent in the liver, where it converts C-H bonds in fat-soluble compounds into C-OH groups (alcohols), which can then be excreted via the kidneys and urinary system. The active intermediate involved in breaking the C-H bond appears to be a terminal iron oxide, Fe=O.
Figure CC13.1. The proposed activated iron center of Cytochrome P450. The heme ring is simplified.
- Oxygen atoms can form double bonds with metals.
- These donors are called "oxide" or "oxo" ligands.
Oxides are also important industrially. Olefin metathesis catalysts, which are used in refining alkenes in petroleum into more useful isomers, are often metal oxides, which are converted under the reaction conditions into metal carbenes (see below).
Problem CC13.1.
Explain, in terms of intermolecular interactions, why oxidation by cytochrome P450 is a necessary step for removal of many compounds from human tissues.
Problem CC13.2.
Show how a metal orbital and a ligand orbital can combine to form a pi bond. How many different transition metal orbitals could participate in this bond?
Problem CC13.3.
Often, "terminal" metal-ligand multiple bonds could be in equilibrium with "bridging" ligands between two metal atoms. Show, with drawings, how the oxide ligands on two adjacent Fe=O groups could form a single Fe2O2 unit.
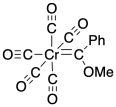
A second important class of metal-ligand multiple bonds is the carbenes. Carbenes contain metal-carbon double bonds. They are often divided into two classes: Fischer carbenes and Schrock carbenes or alkylidenes. Fischer carbenes were developed by E.O. Fischer, who shared a Nobel Prize with Geoff Wilkinson in 1973 for other work. Fischer carbenes have a heteroatom attached to the double bonded carbon, such as an oxygen or nitrogen. They can be somewhat more stable than alkylidenes, which have only hydrogens or carbons attached to the double bonded carbon.
Figure CC13.2. A Fischer carbene complex.
- Carbon can also form double bonds to metal atoms.
- These ligands are called "carbenes".
Problem CC13.4.
Show, with drawings of molecular orbitals, why Fischer carbenes are stabilized by the presence of adjacent heteroatoms.
Alkylidenes were discovered by Dick Schrock, now at MIT, when he was working at DuPont in the early 1970's. While trying to place some bulky alkyl groups on tantalum, he noticed spectroscopic evidence that suggested a double bond. DuPont didn't have him doing this experiment for a particular reason; he was employed as a basic scientist, whose job it was to produce new information for its own sake. However, Schrock quickly realized he had found something with important applications: these kinds of structures had been proposed by Chauvin as intermediates in olefin metathesis, a process used in petroleum refining, but they hadn't been observed before. Years later, Schrock and other workers, including Bob Grubbs at Cal Tech, were able to develop new alkylidene-based catalysts useful in polymer chemistry and organic synthesis. For their contributions in this area, Schrock, along with Bob Grubbs at Caltech and Yves Chauvin at IFP, shared the Nobel Prize in chemistry in 2005.
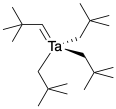
Figure CC13.3. A Schrock carbene or alkylidene complex.
Problem CC13.5.
Suggest some examples of spectroscopic evidence that may have tipped Schrock off about the presence of a metal-carbon double bond in his product.
Problem CC13.6.
Determine the electron count at the metal in the following complexes.
Metal-Metal Multiple Bonds
Just as additional orbital interactions can lead to metal-ligand multiple bonds, they can also make multiple bonds between metals. Metal-metal bonds in coordination complexes are interesting because they are a sort of halfway state between bulk elemental metals, in which arrays of atoms are bonded together more or less without limit, and molecules, which have discrete shapes and sizes. Non-molecular compounds can sometimes be difficult to study (although they also provide some advantages). Sometimes, researchers are interested in the behaviour of compounds having metal-metal bonds because they can provide insight into metals.
It isn't easy to tell how many bonds there are between two metal atoms. Usually, researchers first speculate a multiple bond is present when an x-ray structure shows that the two metal atoms are very close together. Molecular orbital calculations are then performed to get an idea of the electronic structure of the compound. The number of bonds that should be drawn between the two atoms may still be open to debate; most workers draw a bond for every pair of electrons shared between the atoms. However, sometimes there are electrons in antibonding levels as well, so the true bond order between the metals is lower.
Problem CC13.7.
A sigma bond has maximum electron density along the bond axis. A pi bond has maximum electron density above and below the bond axis (electron density is divided into two lobes along the bond). A delta bond has maximum electron density above and below and in front of and behind the bond axis (electron density is divided into four lobes along the bond). Show how two metal d orbitals can combine to form a delta bond.
Problem CC13.8.
Determine the electron count at each metal in the following complexes.
Problem CC13.9.
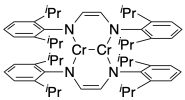
One of the shortest metal-metal bonds on record was reported by Klaus Theopold at the University of Delaware and Clark Landis at the University of Wisconsin-Madison in 2007. The Cr-Cr distance is reported as 1.8028(9) Angstroms (the 9 in parentheses is the error in the last digit, i.e. +/- 0.0009). They believe each chromium atom in the complex has 18 electrons.
a) How many metal-metal bonds are there between the chromium atoms?
b) Show drawings of the overlap between pairs of orbitals that could be responsible for the metal-metal bond.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by
Chris Schaller is licensed under a Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Coordination Chemistry Index
Back to Web Materials on Structure & Reactivity in Chemistry