Reactivity in Chemistry
Electrophilic Addition to Alkenes
EA3. Solvent Participation in Electrophilic Addition
Alkenes can donate their electrons to strong electrophiles and the resulting carbocations combine with the counterion of the electrophile to undergo an overall addition reaction. However, there may be some cases in which the counterion does not combine with the carbocation.
Hydrobrominations of the type we have looked at only occur under certain conditions. Other conditions can lead to other products. For example, a solvent such as water can also participate in the reaction.

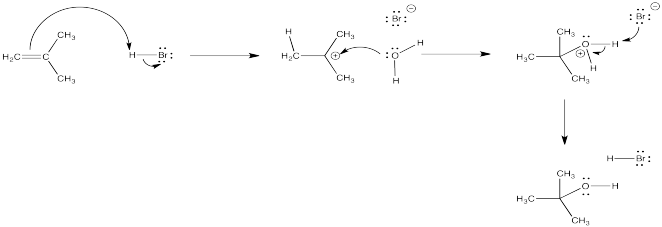
Figure EA3.1. Addition of strong acid in water leading to an alcohol.
The oxygen-based cation (or oxonium ion) that results can easily lose its charge through loss of a proton. As a result, a molecule of water adds to the alkene overall. The alkene becomes an alcohol. This reaction is called an "acid-catalyzed hydration" of an alkene.
Problem EA3.1.
Explain how the hydration of an alkene in the presence of acid is a catalytic reaction.
Problem EA3.2
In many cases, equilibrium mixtures of multiple products may result from the addition of acids to alkenes. Show mechanisms, with curved arrows, for the following reactions.
a) The conversion of 2-butene to 2-chlorobutane with aqueous HCl.
b) The conversion of 2-butene to 2-butanol with aqueous HCl.
c) The conversion of 2-chlorobutane to 2-butene with aqueous HCl.
d) The conversion of 2-butanol to 2-butene with aqueous HCl.
In more concentrated aqueous HBr or HCl, a nucleophilic bromide or chloride ion may still be present in high enough concentration that it could successfully compete with the water for the cation some of the time. A mixture of products could result, including both alkyl halide and alcohol. If someone wanted to make an alcohol from an alkene, they would be more likely to use aqueous sulfuric acid. The sulfate or bisulfate ion is much less nucleophilic than a halide, so there would be less trouble with competing reactions. (Even that does not work well in some cases, so we will see some more sophisticated methods later.)
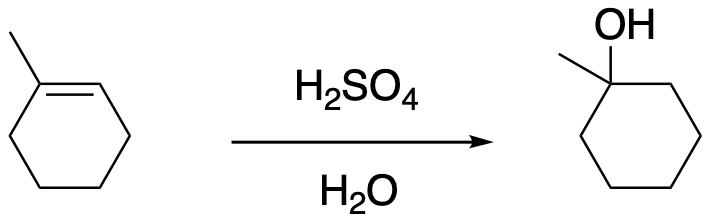
Figure EA3.2. Addition of strong acid in water leading to an alcohol.
On the other hand, in the absence of any solvent, the bromide ion might still have some competition in the second step. The neat reaction (neat means "without solvent" of an alkene with a small amount of acid can result in polymerization. The alkene, which acted as a nucleophile in the first step, can also act as a nucleophile in the second step.
It is important to remember that in any reaction, millions of molecules are involved. Even if one alkene molecule reacts with acid in the first step of a reaction, there are still plenty of other alkene molecules around to act as nucleophile in the second step.

Figure EA3.3. Addition of HBr without any solvent leading to polymerization..
Problem EA3.3.
Provide a mechanism for the polymerization shown above. Assume there are four 2-methylpropene molecules and one hydrogen bromide molecule to begin.
Problem EA3.4.
Chain reactions involve an initiation step, in which a reactive species is generated; propagation steps, in which the reactive species reacts to make a new reactive species; and a termination step, in which the reactive species reacts to make a stable molecule. Label each of the steps in your mechanism from the previous question.
Once again, the nucleophilic bromide would still combine with the cation some of the time, resulting in a mixture of short-chain alkyl halides. Maybe the number of repeating units ranges from 1 to 10, for example. If someone really wanted to make a polymer, the most common method is to add some boron trifluoride, BF3. This compound is a strong Lewis acid. In general, it's difficult to use strong Lewis acids without absorbing small amounts of moisture from the air; that's the trace of water in the equation. The combination of BF3 and H2O forms H+HOBF3-, which provides a proton source without adding a good nucleophile.
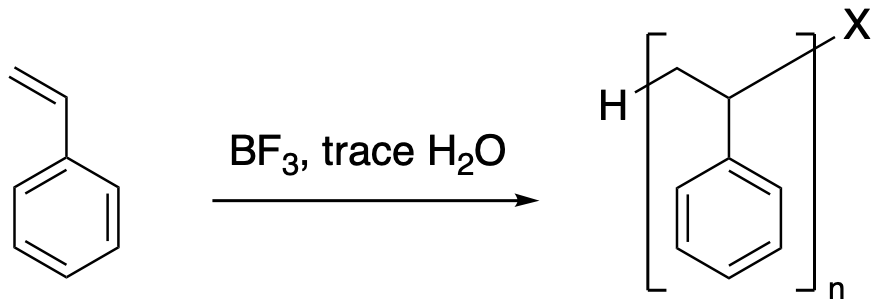
Figure EA3.4. Real conditions for cationic alkene polymerization..
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: