
Reactivity in Chemistry
Electrophilic Addition to Alkenes
EA4. Stabilized Cations in Electrophilic Addition
Electrophilic addition to alkenes generally takes place via donation of the π-bonding electron pair from the alkene to an electrophile. So far, we have only looked at protic electrophiles, but the reaction proceeds with others, as well. For instance, alkenes react quite easily with bromine.

Figure EA4.1. Addition of Br2 across the double bond of an alkene.
Dripping a solution of bromine into a solution of alkene provides a clear sign of reaction. The red-brown colour of bromine disappears almost instantly. The product of the reaction always results from the two bromines adding to opposite faces of the alkene. In the case of cyclohexene, the reaction forms a mixture of (S,S)- and (R,R)- dibromocyclohexane, but no (S,R)-isomer. So, there is a stereochemical story to unpack here. There is also the story of how a Br2 molecule, which has lone pairs and looks like a nucleophile, can be an electrophile for an alkene.
Although bromine isn't an obvious electrophile, most of the common diatomic elements can behave that way; the exception is dinitrogen. A fleeting asymmetry of electrons can polarize the molecules to one end. That event leaves one atom partially positive and the other end partially negative. Because these elements tend to form somewhat stable anions, the partially negative atom can be displaced failry easily.
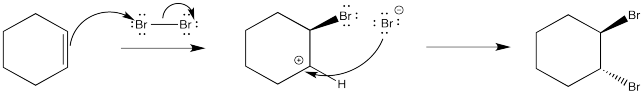
Figure EA4.2. Hypothetical mechanism for bromination of an alkene.
As before, a nucleophile connects with the cation in a second step. In this case, a dibromide compound is formed. However, it isn't formed in quite the way that is shown above. We know that the mechanism shown above does not convey the whole picture because it isn't consistent with the stereochemistry of the reaction shown previously. Remember, the reaction forms the trans-dibromocyclohexane. It does not form cis-dibromocyclohexane. That means the second bromine must approach the cation only from the face opposite the first bromide.

Figure EA4.3. Preferred approach of second bromine.
Problem EA4.1.
Assign configurations R or S to the product shown in the above mechanism.
However, although two enantiomers are formed in the reaction, the corresponding diastereomer is not. The two bromines always add from opposite faces of the alkene. They never add from the same face of the alkene. The following step does not occur.
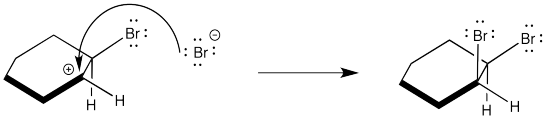
Figure EA4.4. Unobserved approach of second bromine.
Problem EA4.2.
Assign configurations R or S to the product shown in the above mechanism and explain why it is not an enantiomer to the compound in problem EA4.1.
Instead, the cation that forms in the reaction appears to be stabilized by lone pair donation from bromine. The intermediate species below is called a cyclic bromnium ion. The bromine prevents approach of the nucleophilic bromide from one side, ensuring formation of product through anti addition only. The trans product forms as a result.
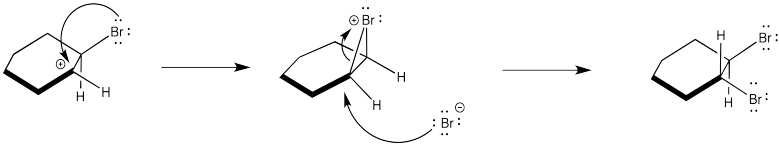
Figure EA4.5. A cyclic bromonium ion forming during the mechanism.
In fact, the cyclic bromonium ion must form in the very first step. There is no evidence of carbocation formation, such as hydride shifts. So, as the alkene donates to the bromine, the bromine lone pair donates back to the developing positive charge on the carbon.
Figure EA4.5. A cyclic bromonium ion forming in the first step of the mechanism.
Problem EA4.3.
Additional evidence of the stabilized bromonium comes from the observation of just one product in the bromination of 1-hexene. How many products would be expected in the absence of a stabilized cation? Explain with a mechanism.
Problem EA4.4.
Bromonium ions like the one shown below have been isolated and characterized by X-ray crystallography in at least one case. Explain why the intermediate was isolated in this case, rather than a dibromo product.
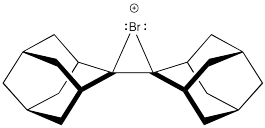
Problem EA4.5.
In some cases, the reaction of an alkene with bromine does not provide dibromo products. Show products of the reaction of cyclohexene under the following conditions and justify your choices with mechanisms.
a) Br2 in water b) Br2 and NH4+ Cl- in THF
Problem EA4.6.
Provide products for the reaction of bromine with each of the following compounds.
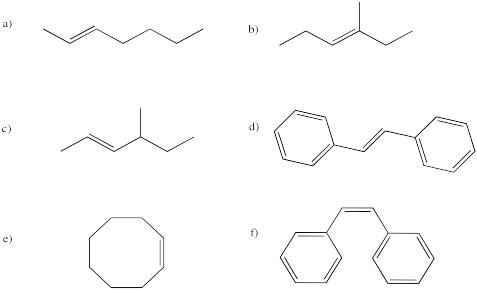
As we saw before under acidic conditions, the reaction of bromine with alkenes can be strongly dependent on the solvent. Whereas reaction in organic solvents allows the bromide anion to connect with the cyclic bromonium ion and produce a 1,2-dibromo compound, reaction in water takes a different course. The product is sometimes called a "bromohydrin". In a bromohydrin, there is a bromine on one carbon and a hydroxy group on the carbon next to that. Chlorohydrins are very similar, with a chlorine on one carbon and a hydroxy group on the next one. In general, both kinds of compounds are sometimes called halohydrins.
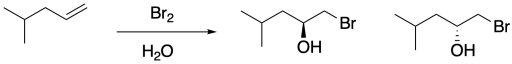
Figure EA4.7. Participation of solvent (water) during bromination.
Once again, this product results from the water acting as a nucleophile rather than the bromide ion. Remember, if water is the solvent, the bromonium ion intermediate is surrounded by water molecules, so it is likely that a water molecule connects with the bromonium ion before the bromide can get in the proper position to react. That addition of water results in the installation of a hydroxy group to one of the carbons of the cyclic bomonium ion.
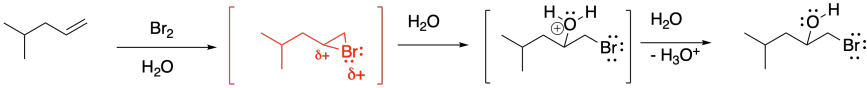
Figure EA4.8. Mechanism showing regiochemistry of bromohydrin formation.
In general, the water adds to the most-substituted carbon in the cyclic bromonium ion, placing the hydroxy group on the most-substituted position of the former alkene. That's because the positive charge in the cyclic bromonium ion is shared between the bromine and the more-substituted carbon. Cations are relatively stable on more-substituted carbons, so the positive charge is most stable when it is partly distributed on bromine and partly on that more-substituted carbon. However, that makes the more-substituted carbon susceptible to donation by a nucleophile; in this case, that's the water.
Just as we saw in regular bromination, if the product of this reaction has two new chiral centres, then it is apparent that the bromine and the hydroxy group are trans to each other. Most often, the reaction still produces a pair of enantiomers, because there is not usually anything in the reaction conditions to favour one enantiomer over the other, but both enantiomers display that trans relationship. The cis diastereomers are not observed.
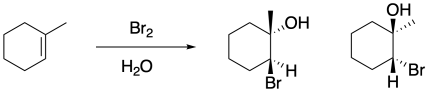
Figure EA4.9. Stereochemistry of bromohydrin formation.
The reason for this diastereoselectivity is the same as the controlling factor in regular bromination. The nucleophile approaches the cyclic bromonium ion from the face opposite the bromine (an anti-addition), resulting in a trans-relationship between the hydroxy group and the bromine. That approach results from the bromine blocking one face of the molecule, so the nucleophile must approach from the other face.
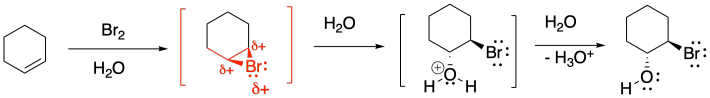
Figure EA4.10. The reason for stereochemical control of bromohydrin formation.
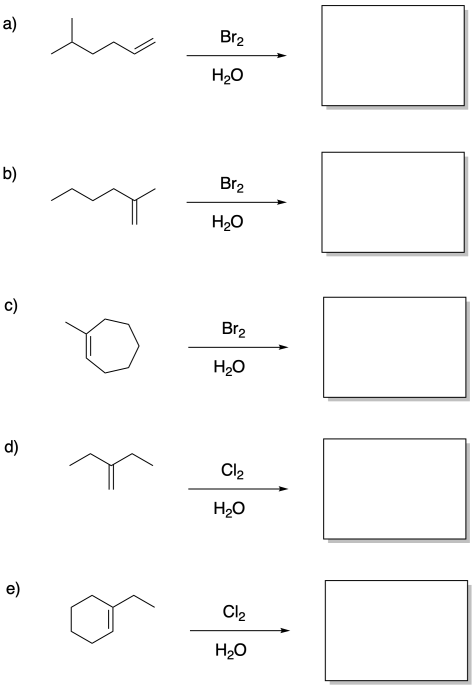
Problem EA4.7.
Draw products of the following reactions.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: