Part MI1. Introduction
Insertions constitute a class of reactions involving transition metals. These reactions are important in some industrial processes that are catalyzed by transition metals. One of the most important processes that involves these reactions is hydroformylation. Hydroformylation is used to convert unsaturated hydrocarbons into aldehydes. These long-chain aldehydes are then converted into important commodities, such as detergents and fragrances.
There are two basic kinds of insertions: migratory insertions (or 1,1-insertions, more generally) and beta-insertions (or 1,2-insertions).
-
Migratory insertions are related to the addition of nucleophiles to carbonyls.
-
Migratory insertions involve transition metal compounds that bind to a carbon monoxide.
-
The transition metal is bound to another group, such as an alkyl group or a hydride.
-
The alkyl or hydride is transferred from the metal to the carbon of the bound carbon monoxide (figure MI1.1).
Figure MI1.1. A general migratory insertion (or 1,1-insertion) reaction.
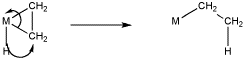
The other type of insertion, 1,2-insertion, often involves alkenes, or other ligands that can bind to a metal through two atoms instead of just one (Figure MI1.2).
Figure MI1.2. A general 1,2-insertion of an alkene.
Note that, in transition metal chemistry, formalisms are often used differently than in simple main group compounds involving carbon, oxygen and nitrogen..
-
Sometimes, formal charges are not shown.
-
Non-bonding electrons on metals are rarely shown.
Structures are often complicated enough that it becomes difficult to draw all the non-bonding electrons and formal charges and still have a clear picture. People who work in this area will keep count of electrons in their head, although they will frequently jot down the electron count on the metal beside the structure in order to keep track.