Reactivity in Chemistry
Reaction Kinetics
Contribution from Brian Johnson, College of Saint Benedict / Saint John's University
MK1. Determination of Activation Parameters
Intuitively, you know that a reaction goes faster as the temperature is raised, as more reactant molecules have the energy needed to overcome the activation barrier to the reaction. The Arrhenius equation relates reaction rate constants (k) and temperature. One of the forms of the Arrhenius equation is:
ln k = -Ea/RT + ln A
where Ea is the activation energy for the reaction, T is the absolute temperature (in Kelvin) at which a corresponding k is determined, R is the gas constant, and A is a pre-exponential factor. The activation energy may then be extracted from a plot of ln k vs. 1/T, which should be linear. This plot is called an "Arrhenius plot".
Problem MK1.1.
Recall that y = mx + b.
a) In this "Arrhenius plot", what is the slope?
b) What is the intercept?
In practice, activation energies are not often cited in the current literature. Instead, a similar but more useful equation called the Eyring equation is used. The Eyring equation is:
ln (k/T) = -ΔH‡ /RT + ln (kB/h) + ΔS‡ /R
where k, T and R are the same as in the Arrhenius equation, kB is Boltzmann�s constant, h is Planck�s constant and ΔH‡ and ΔS‡ are the enthalpy and entropy of activation, respectively.
A plot of ln(k/T) vs. 1/T gives a stright line fitting the equation: y = mx + b.
m = -ΔH‡/R or ΔH‡ = -mR
R = 8.314 J K-1 mol2-1 = 38.3 kJ mol-1
b = ΔS‡/R + ln(kB/h)] or ΔS‡ = 8.314 * [b - ln(kB/h)]
kB = 1.38 x 10-23 J K-1 and h = 6.626 x 10-34 J s
Problem MK1.2.
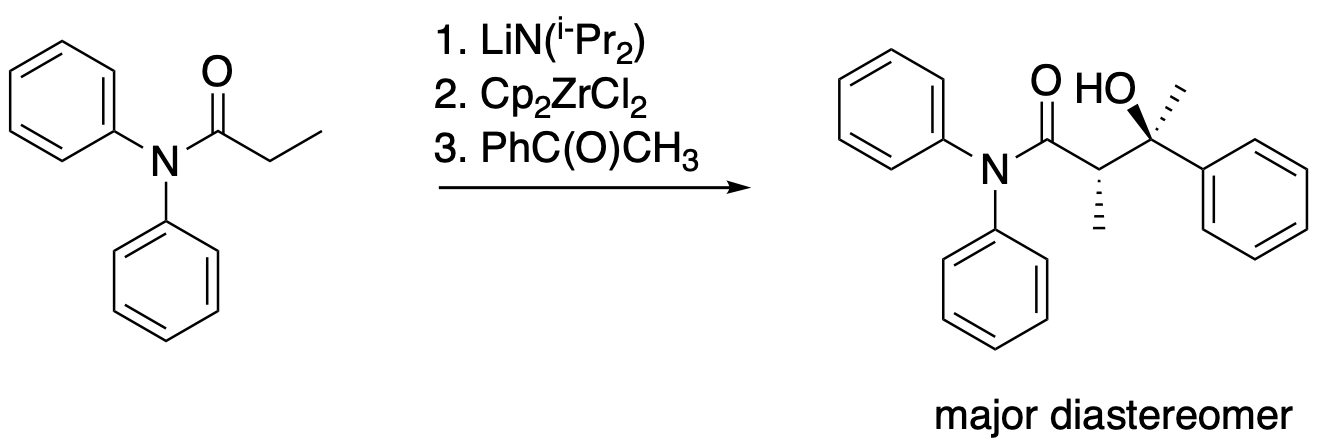
The following protocol for a modified aldol reaction was reported by the Floriani lab at the University of Lausanne, Switzerland, with collaborators at the University of Parma, Italy (Cozzi, P.G.; Veya, P.; Floriani, C.; Rotzinger, F. P.; Chiesi-Villa, A.; Rizzoli, C. Organometallics 1995, 14, 4092-4100):

Kinetic data across a range of temperatures gave the following plot:
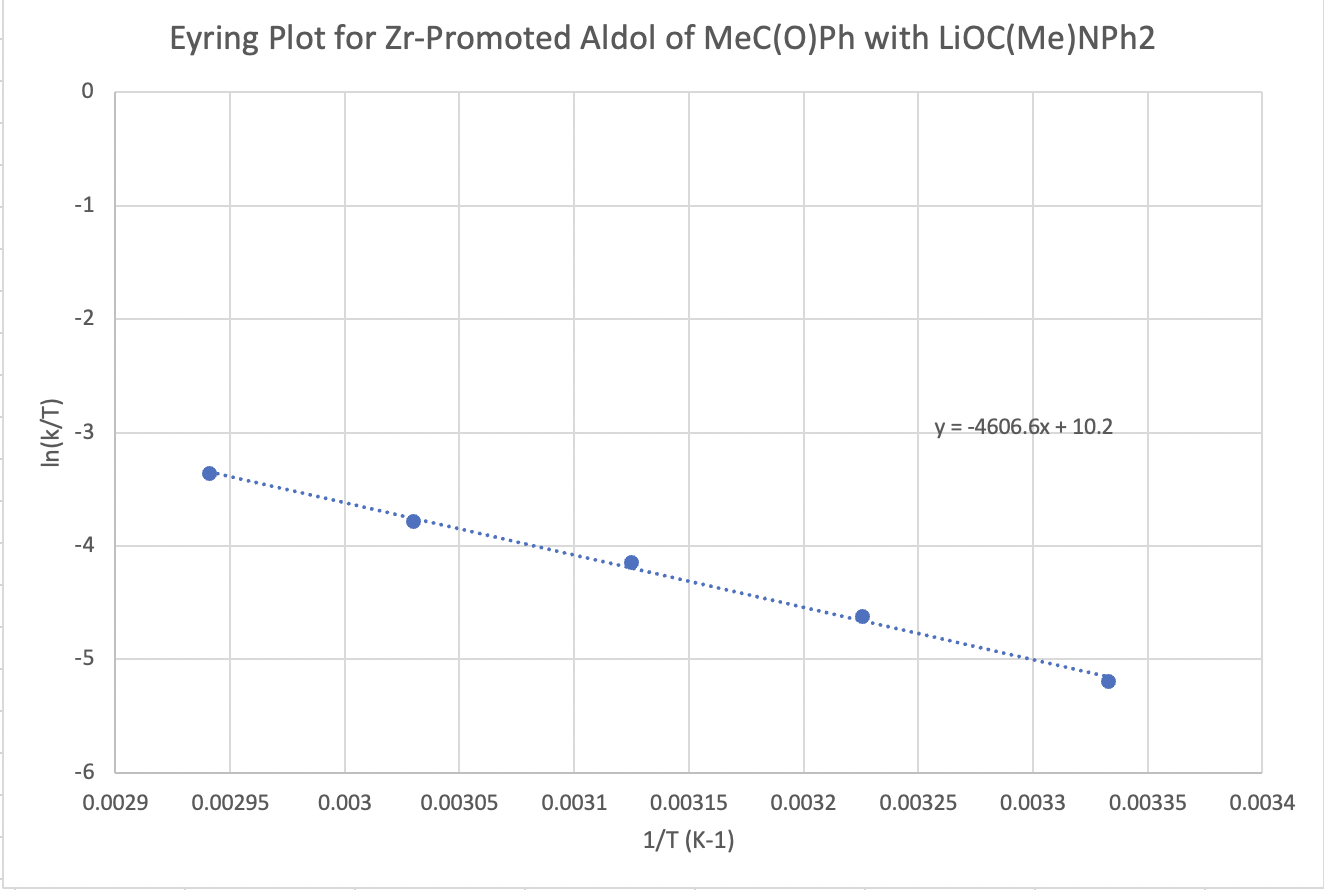
Calculate the enthalpy and entropy of activation for the reaction.
Problem MK1.3.
The following protocol for a modified aldol reaction was reported by the Shapley lab at the University of Kansas (Mitton, C.G.; Schowen, R.L.; Gresser, M.; Shapley, J. J. Am. Chem. Soc. 1969, 91(8), 2036-2044):
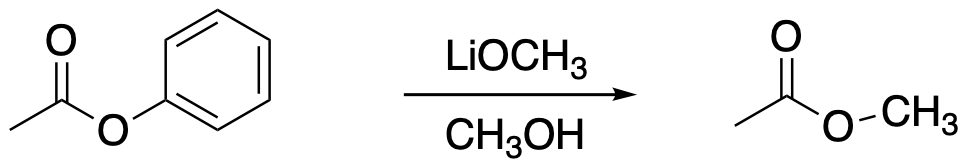
Kinetic data across a range of temperatures gave the following plot:
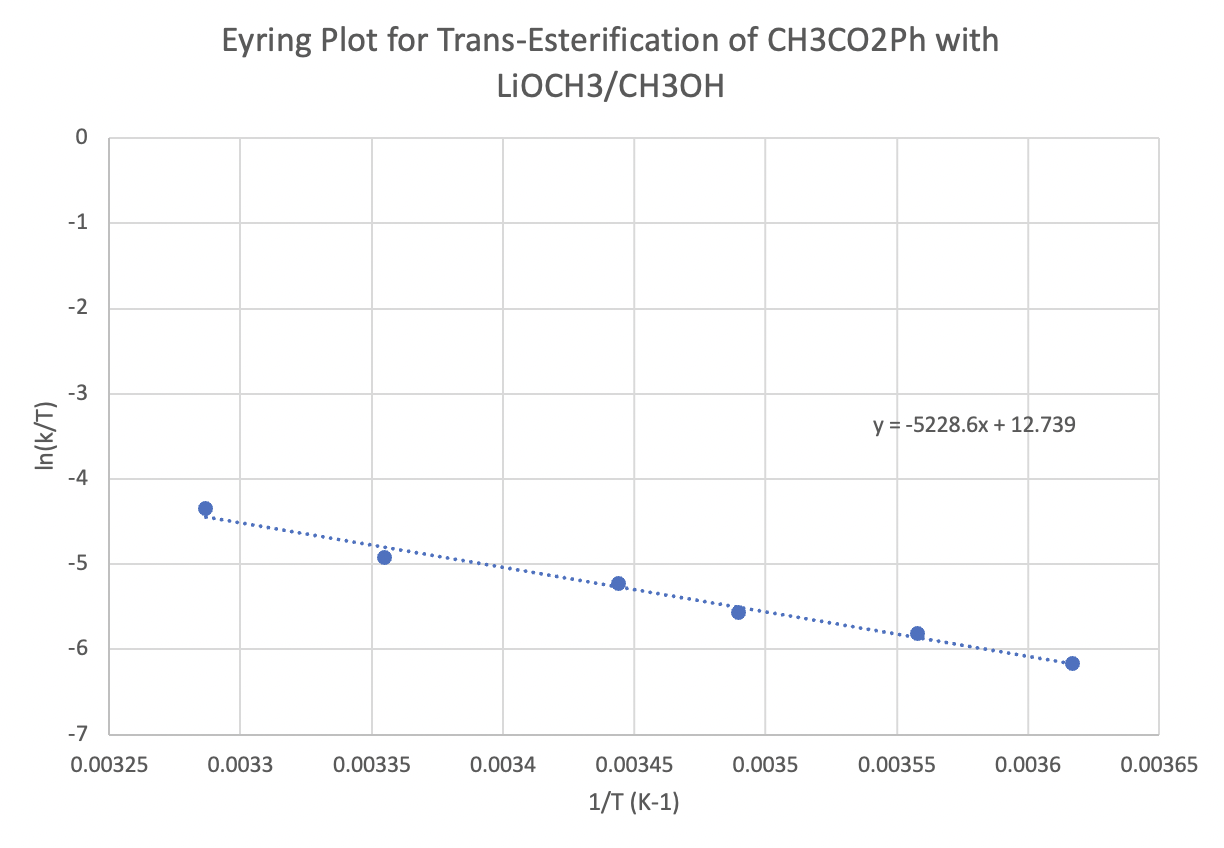
Calculate the enthalpy and entropy of activation for the reaction.
Problem MK1.4.
Using the following data, construct an Eyring plot and determine the activation enthalpy and entropy.
|
1/T (K-1) |
ln k (unitless) |
| 0.00152 | 3.7 |
| 0.00157 | 3.2 |
| 0.00160 | 2.9 |
| 0.00165 | 2.2 |
| 0.00170 | 1.6 |
Problem MK1.5.
Using the following data, construct an Eyring plot and determine the activation enthalpy and entropy.
| T (°C) | k (mol L-1 s-1) |
| 40 | 1.3 x 10-4 |
| 50 | 2.2 x 10-4 |
| 60 | 4.0 x 10-4 |
| 70 | 7.5 x 10-4 |
| 80 | 1.4 x 10-3 |
Note that the activation parameters (ΔH‡ and ΔS‡ ) are not the same as the entropy and enthalpy of the reaction, which can usually be calculated from tables of values. Since they depend on how the reaction proceeds, not just the initial and final states of the reaction, they must be determined experimentally. Once that has been done, interpretation of the numerical values provides insight into the mechanism of the reaction.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Mechanistic Determination
Back to Web Materials on Structure & Reactivity in Chemistry