Reactivity in Chemistry
Reaction Kinetics
RK10. Catalysis
The mechanism of a reaction is a sequence of elementary steps leading from the starting materials to a series of intermediates and eventually to the products. Each step involves an activation barrier. Each intermediate has some measure of stability. We can keep track of energy changes along this reaction pathway by using a reaction progress diagram.
We can change the speed of a reaction by flooding the system with reactant or by adding more energy to help it get over the barrier, but the reaction still follows this same energy pathway.
However, if we add a catalyst, that's no longer true. A catalyst introduces a completely different pathway that wasn't there before.
Some reactions simply can't proceed without a catalyst. There is too high a barrier to proceed. That may be true even if the overall reaction is exergonic and should be favoured to proceed.

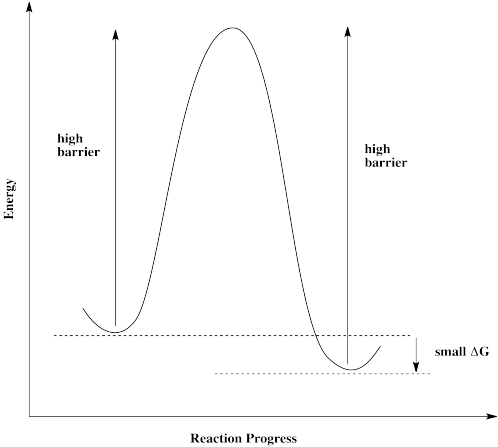
Figure RK10.1. A reaction barrier.
If molecules approach this barrier from the left, they encounter a very large barrier. They can't proceed, even though it would be energetically favourable for them to get to their destination. The molecules get stuck.
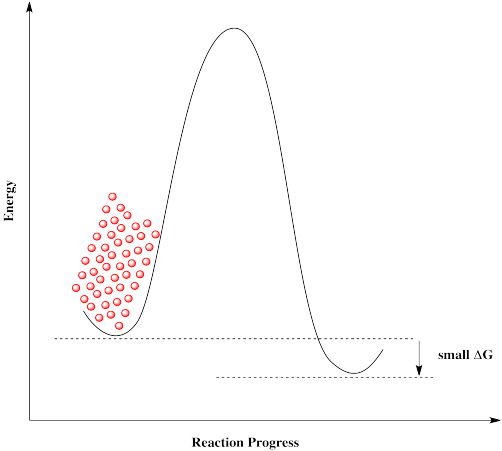
Figure RK10.2. A reaction barrier preventing molecules from reacting.
The same is true if molecules approach from the right. They can't get over the barrier, even though thermodynamically their destination is just a short hop. These molecules are stuck.
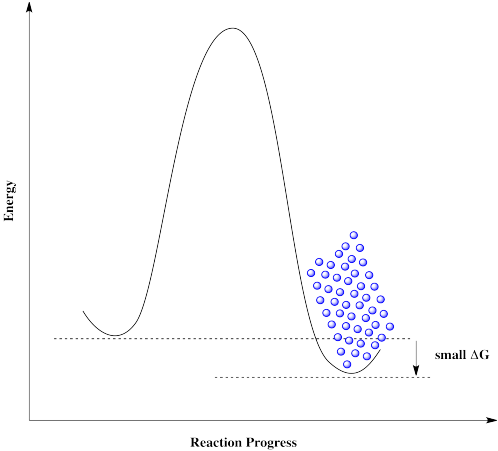
Figure RK10.3. A reaction barrier preventing the reverse reaction.
Everything would be fine if we could just get rid of that darned barrier. The molecules could freely move back and forth, and settle out where they are supposed to be.
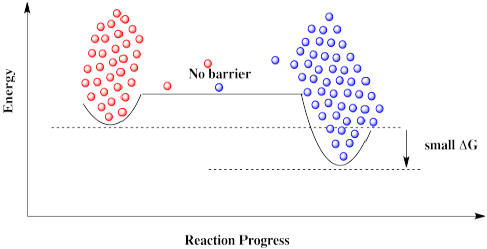
Figure RK10.4. Without that high barrier, the reaction can proceed in either direction.
A catalyst doesn't remove the barrier, but it offers a new pathway with a lower barrier. The reaction is able to proceed back and forth.
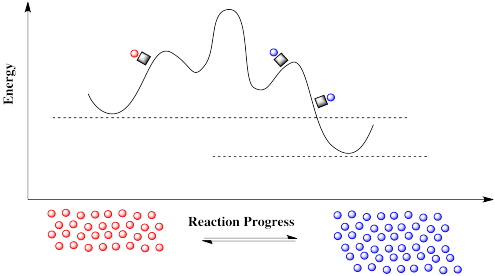
Figure RK10.5. A reaction under catalysis may require additional steps, but has lower reaction barriers.
How can there suddenly be a new pathway? A reaction progress diagram plots what happens to the energy along one particular coordinate of interest to the molecule. We may be following a bond as it lengthens and breaks through the course of a reaction. But there are always other energetic possibilities that remain unseen in this diagram. What we are looking at is merely a slice through a potential energy surface. A potential energy surface is like a landscape, a mountain range in which elevation corresponds to energy. The reaction progress diagram depicts a single pathway from one energetic valley to another. In one of these valleys is the reactant; the product is in the other. The path from one valley to another leads uphill, over a mountain pass, and down into the other valley again.
Suppose you are going to visit a friend. You live in one valley and the friend lives in another. Every day you walk the same path to your friend's house. You aren't a fool, so you take the easiest route, over the lowest mountain pass.
One day, instead of visiting our friend on foot, we are going to take the train. The train takes a completely different pathway than the one we are used to. It doesn't even go over the same mountain pass; it may take another route that was inaccessible by foot, or it may simply tunnel through the mountain. Furthermore, when the train reaches its destination, it picks up more passengers and makes the same journey again, over and over.
In molecular turns, it is the reactant that takes the train, a low-energy pathway, on its way to the product. That train is a catalyst, and it has some very important features.
That recycling of the catalyst is sometimes referred to as "turnover". The turnover number of a catalyst is the number of times the catalyst is able to return and carry out the reaction again. (Eventually something may go wrong and the catalyst may stop working.) The reactant in a catalytic reaction is often called the substrate, particularly in cases in which the catalyst is an enzyme of a transition metal catalyst. The speed at which the catalyst is able to carry out the reaction on new substrates is called the turnover frequency. These are important parameters in describing the efficiency of a catalyst.
Problem RK10.1.
Polymerization catalysts take small molecules called monomers and connect them together using the same reaction over and over again to make a long polymer chain. What was the minimum turnover number of the catalyst used to make each of the following polymers? (Mn = number average molecular weight, a statistical estimate of the average size of polymer chain.)
a) polylactide, Mn = 3,000, from lactide, C6H8O4.
b) polystyrene, Mn = 250 thousand, from styrene, C6H5C2H3.
c) high-modulus polyethylene (HMPE), Mn = 6 million, from ethene, C2H4.
Problem RK10.2.
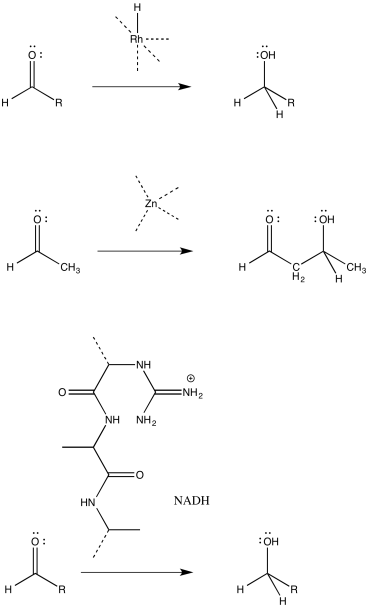
Catalysis often begins with a binding step, followed by one or more subsequent steps needed to carry out the reaction. For the following catalysed reactions, draw the binding step using curved arrows.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Web Materials on Structure & Reactivity in Chemistry