Reactivity in Chemistry
Nitrogen Reduction
NF2. The Haber-Bosch Process
The Haber-Bosch Process is one of the world's most important industrial reactions. It provides for the synthesis of ammonia directly from elemental nitrogen, N2, and hydrogen, H2. Since its development in the early twentieth century, it has led to the production of an enormous quantity of fertilizer, vastly increasing global food production. As a result, it is estimated that a significant fraction of the nitrogen content in the typical human body is ultimately derived from this process.
At the time of its development, the Haber-Bosch Process supplanted a growing dependence on guano, seabird droppings, harvested in order to enrich farmland. Although this method had been practiced by the Inca for centuries, European demand in the late 1800's placed growing pressure on resources in Peru and the Caribbean. Because nitrogen is a limiting factor for plant growth, treatment of soil with nitrogen- and phosphorus-rich guano led to remarkable improvement in crop yields.
In the late 1700's, Henry Cavendish had been able to produce nitrates with an electric arc in air. A Norwegian development, the Birkland-Eyde Process, harnessed the hydroelectric power available in that country in order to scale Cavendish's feat to industrial production, but the reaction was still terribly inefficient. Fritz Haber was able to develop a much more successful procedure for ammonia production (the reduced state, rather than the oxidized nitrates), which was adopted by BASF. Originally the local Baden Aniline and Soda Factory, this German company is now the world's biggest chemical producer.
Haber himself was a fascinating and controversial figure who hoped to improve the human condition through industrial contributions to agriculture; he also developed pesticides. However, he was roundly condemned for developing and implementing chlorine gas against Allied troops during World War I. Although Haber, who was Jewish, died before the Holocaust, many members of his extended family were murdered in concentration camps; in some of these camps, internees were executed by poison derived from Haber's pesticides.
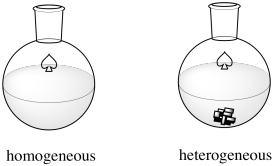
The Haber-Bosch Process is an example of heterogeneous catalysis. Many catalysts operate via homogeneous catalysis, in which the reaction occurs in one phase (in solution). In heterogeneous catalysis, the reaction occurs at the interface between two phases. In this case, the two phases are the gas phase and the solid phase. The reactants, hydrogen and nitrogen, are both gases. They are both introduced into a vessel under enromous pressure and high temperature. The catalyst is a carefully prepared iron oxide supported on a mixture of other metal oxides, although ruthenium and osmium variations have also been used and offer some advantages.

Figure NF2.1. A homogeneous catalyst dissolves. A heterogeneous catalyst does not.
In this case, the reaction involves gas phase reactants and a solid catalyst. The reaction takes place on the surface of the catalyst. We might depict such a surface as a row of atoms. The reaction takes place only on the surface of the atoms (for example, along the top of the picture below). Rows of atoms beneath the surface would not necessarily contribute to the reaction. In the picture below, the second row of atoms might play no role at all in the reaction, other than to provide a place for the top row of atoms to sit.
![]()
Figure NF2.2. A cartoon of a layer of atoms at the surface of a metal (and another layer of atoms beneath them).
It might be more convenient to just think of the reaction happening on a flat surface of metal, ignoring its makeup of atoms.

Figure NF2.3. An even simpler cartoon of a metal surface.
So, molecules come along and they react on that surface. The mechanisms by which molecules react at the surface may be familiar to us. We might find parallels in organometallic chemistry. The surface is, after all, made of metal atoms.
Hydrogen molecules probably become activated through oxidatve addition.
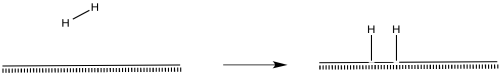
Figure NF2.4. Hydrogen becoming chemisorbed on a metal surface. The hydrogen is shown undergoing oxidative addition to form two hydrides.
Nitrogen molecules must bind at the surface, too.
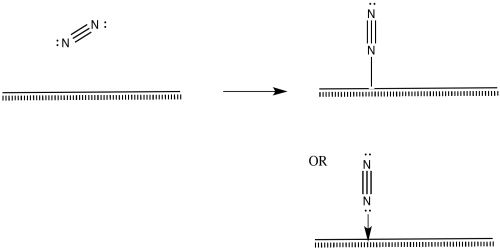
Figure NF2.5. A nitrogen molecule coordinating to the metal surface.
The nitrogen is depicted as "end-bound" above, using its lone pair to attach to the metal. It is also possible that the molecule leans over and becomes "side-bound".

Figure NF2.6. A different mode of nitrogen coordination to the metal surface.
How do the nitrogen and hydrogens react further to make ammonia? There are different possibilities. For instance, a 1,2-insertion reaction seems possible.
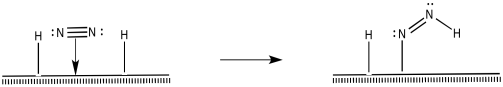
Figure NF2.7. A 1,2-insertion turns coordinated dinitrogen into a diazenyl ligand.
After that, a reductive elimination would result in formation of a second N-H bond.
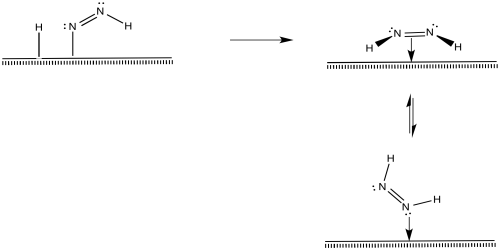
Figure NF2.8. A reductive elimination turns the diazenyl ligand into a coordinated diazene molecule.
That process, so far, results in the conversion of dinitrogen to diazene, N2H2. The diazene could react further to make hydrazine (N2H4) and, ultimately, ammonia.
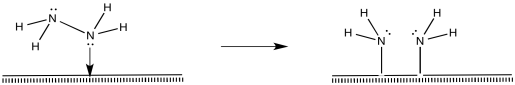
Figure NF2.9. Oxidative addition of a coordinated hydrazine results in two amide (-NH2ligands).
Of course, the reaction could happen in other ways, too. There are surface studies that suggest the presence of nitrides, imides and amides (M=N, M=NH, M-NH2). The presence of nitrides suggests a series of oxidative additions starting with dinitrogen, proceeding all the way to nitride, N3-.
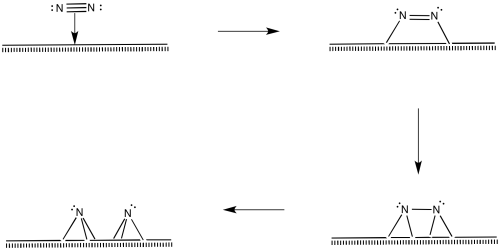
Figure NF2.10. Oxidative addition of dinitrogen, eventually forming two nitride ligands.
Once the nitride is in place, N-H bonds could form via reductive eliminations. The first reductive amination would result in an imide, the second would produce an amide, and the third would produce ammonia.
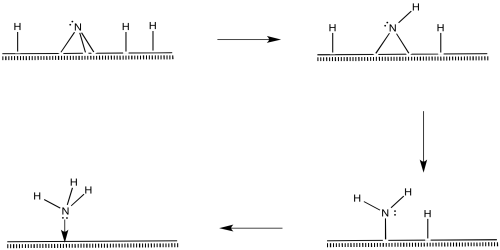
Figure NF2.11. Reductive elimination to form an imide ligand (=NH2), followed by an amide ligand and then coordinated ammonia.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Navigation: