Reactivity in Chemistry
Nitrogen Reduction
NF6. Solutions for Selected Problems
Problem NF1.1.
a) 0 b) 3- c) 3- d) 3+ e) 5+
Problem NF1.2.
a) O2 b) NO2- c) 2 e-
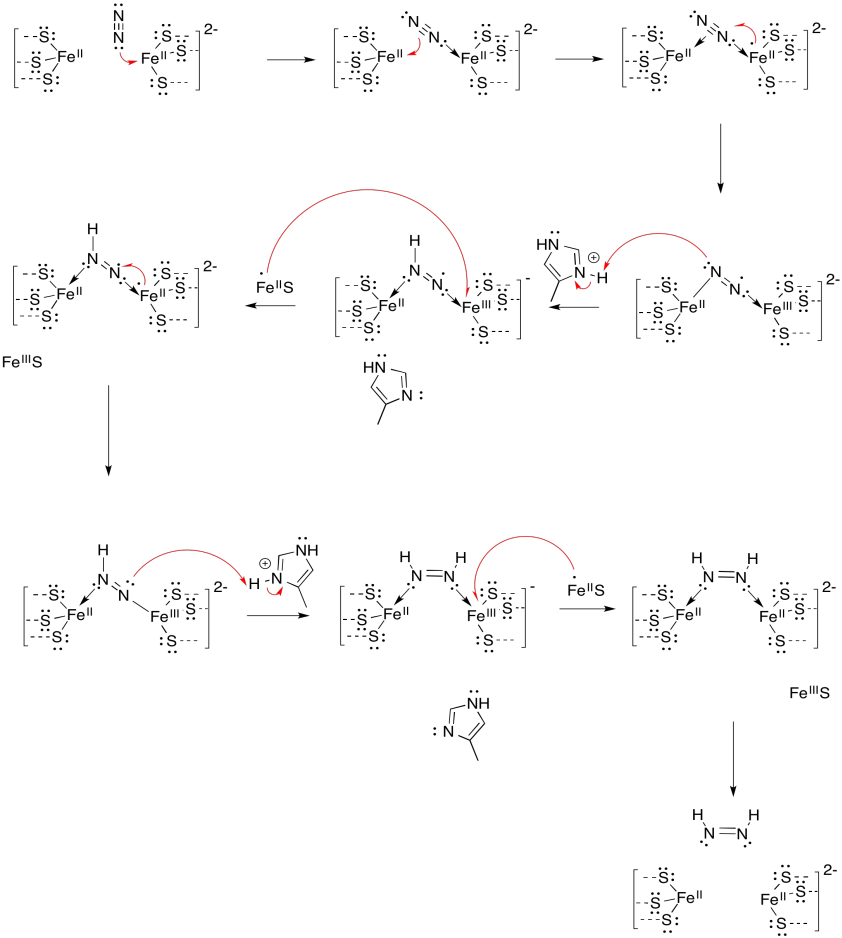
Problem NF3.1.
a) S: 4 x 2- = 8- ; CysS: 4 x 1- = 4- ; Fe: 2 x 2+ + 2 x 3+ = 10+ ; total = 2-
b) S: 4 x 2- = 8- ; CysS: 3 x 1- = 3- ; Fe: 1 x 2+ + 2 x 3+ = 8+ ; total = 3-
c) S: 2 x 2- = 4- ; CysS: 4 x 1- = 4- ; Fe: 2+ + 3+ = 5+ ; total = 3-
Problem NF3.2.

Problem NF3.3.

Problem NF3.4.

Problem NF4.1.
The phosphine is an ordinary sigma donor, but the thioether is a pi donor as well, which would significantly change their complexes.
Problem NF4.2.
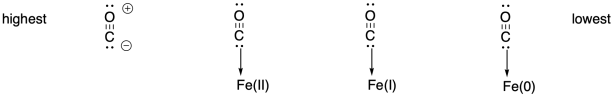
This trend reflects the highest amount of backbonding in the Fe(I) complex. In fact, it could be argued that all of these species become Fe(II) complexes, with a neutral, anionic, and dianionic CO ligand as we proceed from left to right (Wolczanski, P. T. Organometallics 2017, 36, 622−631).
Problem NF4.3.
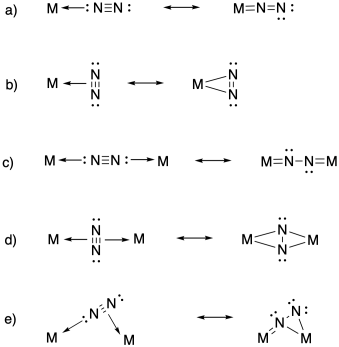
Problem NF4.4.
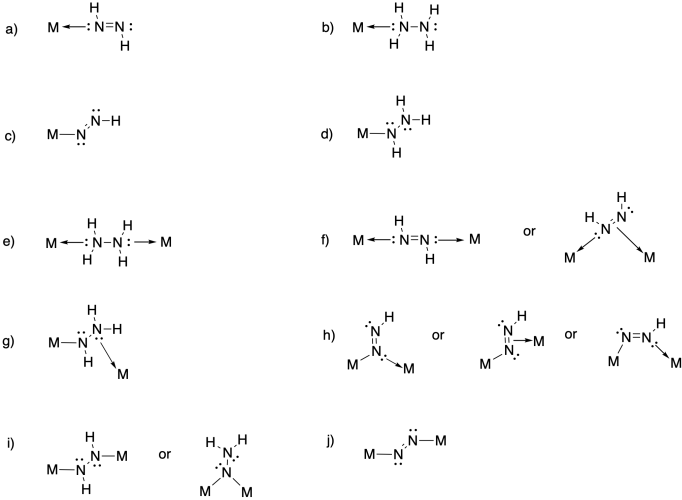
a) η1-dinitrogen
b) η2-dinitrogen
c) μ2-η1:η1-dinitrogen
d) μ2-η2:η2-dinitrogen
e) μ2-η1:η2-dinitrogen
Problem NF4.5.
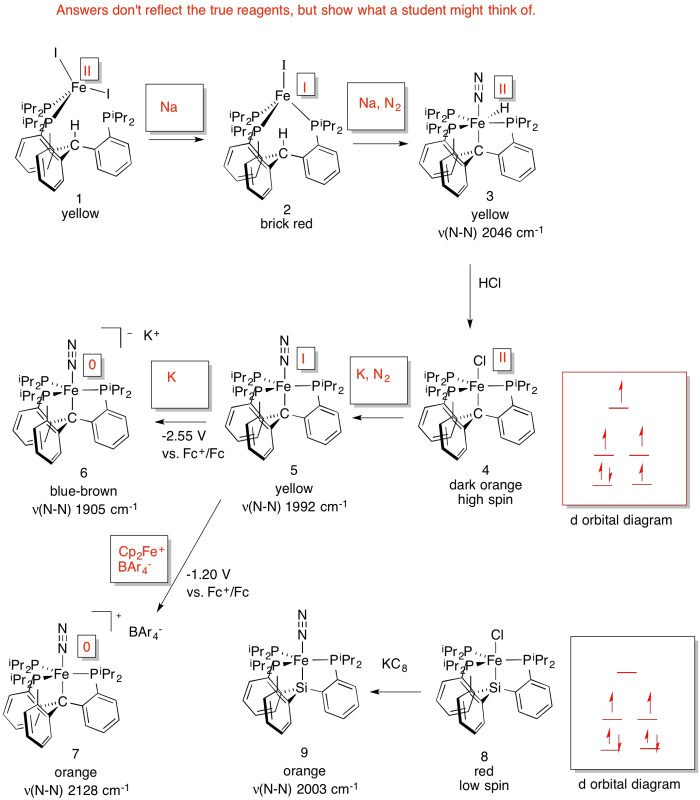
d) As the Fe becomes more reduced, the N-N stretching frequency decreases. That's because the more electron density there is on the Fe, the more it is able to backbond to the N2 (N2 is a π acceptor).
Problem NF5.1.
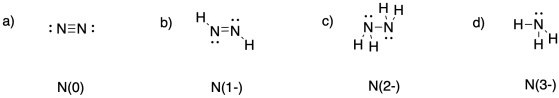
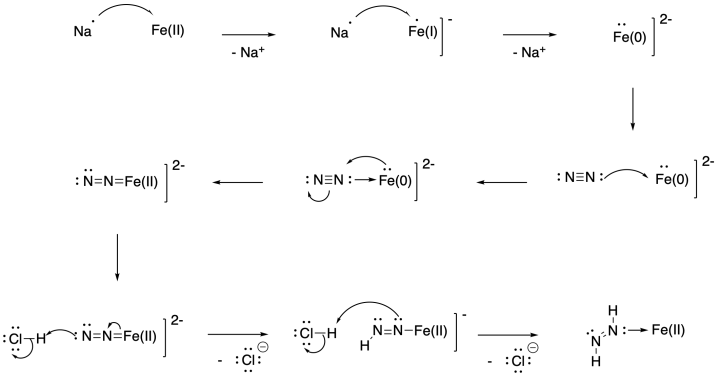
Problem NF5.2.
Problem NF5.3.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Navigation: