
Reactivity in Chemistry
Aliphatic Nucleophilic Substitution
NS2. Mechanism of Aliphatic Nucleophilic Substitution
Aliphatic nucleophilic substitution clearly involves the donation of a lone pair from the nucleophile to the tetrahedral, electrophilic carbon bonded to a halogen. We might expect this carbon to be electrophilic because of the halogen attached to it. Consequently, it attracts a nucleophile. In addition, carbon-halogen bonds can sometimes be relatively weak. As a result, there can be a thermodynamic driving force for replacing that C-X bond for another, stronger bond. Furthermore, halide ions are generally pretty stable, because of both electronegativity and polarizability reasons. Polarizability is the most important factor within the group, so an iodide ion is generally considered more stable with respect to an ionization reaction compared to a fluoride ion. For all of these reasons, it may not be surprising to see nucleophiles replacing halogens in haloalkanes. Chloroalkanes, bromoalkanes, and iodoalkanes all undergo this reaction easily, although fluoroalkanes do not.
| Table NS2.1. Some bond dissociation energies relevant to nucleophilic substitution. | |
| Bond | BDE (kcal/mol) |
| C-C | 87 |
| C-N | 79 |
| C-O | 90 |
| C-F | 115 |
| C-Cl | 84 |
| C-Br | 72 |
| C-I | 58 |
Problem NS2.1.
Compare the electronegativity of carbon to that of fluorine, chlorine, bromine and iodine.
a) On this basis alone, explain why the carbon attached to the halogen would be electrophilic.
b) Which compound should be most electrophilic based on electronegativity: fluoromethane, chloromethane, bromomethane or iodomethane?
c) Use the following bond strengths to estimate the qualitative trend in how easily each bond might undergo substitution: C-F; C-Cl; C-Br; C-I.
d) Fluorocarbons are quite stable towards aliphatic nucleophilic substitution; in general, they do not undergo this reaction. Explain why.
The exchange of a cyanide for a chloride is just one example of a substitution reaction. This reaction turns a chloroalkane into a nitrile. It could be that this reaction is favoured because a weaker carbon-chlorine bond is exchanged for a stronger carbon-carbon bond, although the difference in bond strengths isn't that large. The stability of the chloride ion is another reason this reaction proceeds.

Figure NS2.1. Nucleophilic substitution of cyanide for chloride in 2-chloropropane.
So, a cyanide ion can replace a chlorine attached to a carbon chain. Exactly how the reaction happens is a different question. What is the mechanism for the reaction? By mechanism, we mean the sequence of individual steps that have to happen in order for the reaction to occur. What bonds have to break? What bonds have to be made? Both bond-making and bond-breaking involve the movement of electrons from one atom to another. Which atoms are donating electrons? Which atoms are accepting electrons?
In considering possible mechanisms for this reaction, we ought to think about overall bond-making and bond-breaking steps. In the addition of sodium cyanide to alkyl chloride to make an alkyl nitrile, there is one bond-making step (the C-C bond) and one bond-breaking step (the C-Cl bond). The simplest reaction mechanism would involve some combination of these steps.
Two possibilities immediately come to mind:
Mechanism A
The C-C bond forms and then the C-Cl bond breaks.
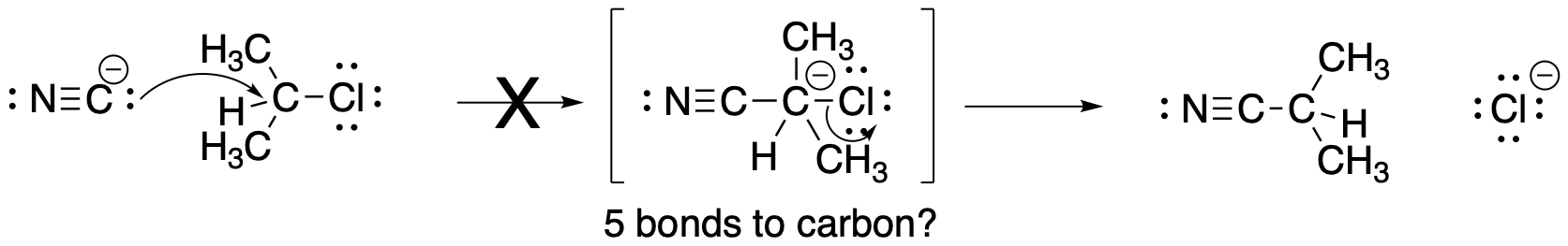
Figure NS2.2. An unlikely mechanism for nucelophilic substitution.
Mechanism B
The C-Cl bond breaks and then the C-C bond forms.
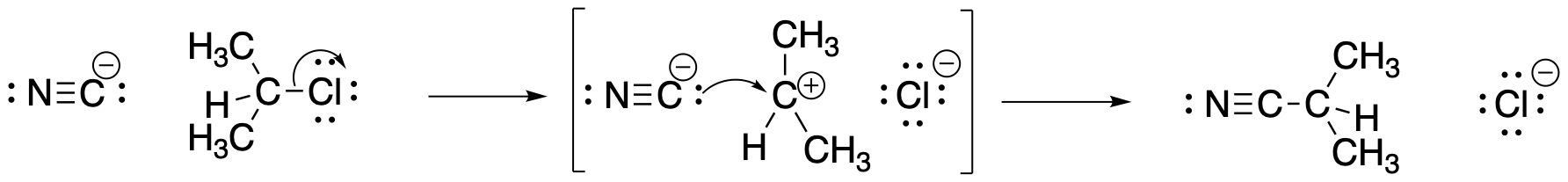
Figure NS2.3. A mechanism for nucleophilic substitution with ionic intermediates.
However, some familiarity with bonding in the second row of the periodic table may suggest to you that mechanism A is not very likely. That mechanism would require forming five bonds to carbon before the C-Cl bond eventually breaks. We can safely ignore this possibility.
Instead, there may be a third possibility to consider.
Mechanism C
The C-Cl bond breaks and the C-C bond forms at the same time.

Figure NS2.4. A concerted mechanism for nucleophilic substitution.
Mechanism C is a concerted mechanism; two bond-making and -breaking events happen at once. However, no octet rules are violated.
Very often when we are thinking about chemical reactions, it is useful to think about the energetics involved with the reaction, and with each elementary step. Is the overall reaction endothermic or exothermic? That is, does the reaction require an overall input of energy to get to the products, or does it give off more energy than it takes in? Is each elementary step endothermic or exothermic? We often graph the changes in energy of the reactants as they proceed to different intermediate stages and finally to the product. The result is a kind of roller coaster cartoon called a reaction progress diagram. You can think about the reactants as riders in this roller coaster, first getting pulled uphill before they are released to rise and fall all the way to the end of the reaction. It's kind of a rough ride, though, so some of their pieces may get mixed up with those of other riders by the time they get to the end.
Reaction progress diagrams for these two reactions would look like the illustrations below. Mechanism B was the label we used for ionization and then addition of the nucleophile:
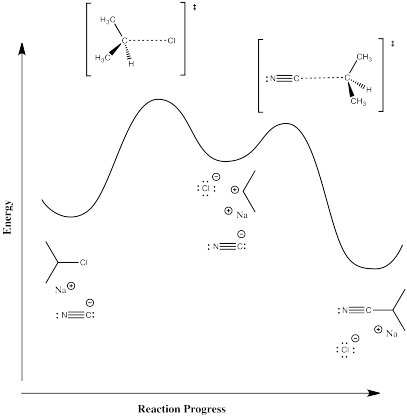
Figure NS2.5. Reaction progress diagram for a 2-step reaction.
This one requires some input of energy to break the carbon-halogen bond. That's the rising hill at the beginning. Now, if we are correct in thinking there is a pair of ions formed in that elementary step, then those ions must have some level of stability. Otherwise, they wouldn't be able to form in the first place. There must be factors, such as the polarizability of the chloride ion, that offer some stability. That little bit of stability means energy goes down just a little bit when the ions form; there is a little valley at the top of the hill where the ions exist. However, these intermediates must be less stable than the reactants or products; otherwise, the reaction would never happen, because everything would just turn into those intermediates and never change. So, the intermediates are in that little valley, high above the reactants and products on either side.
It is often very surprising to students that there is another hill leading from the intermediates to the products. We're not breaking any bonds when we form the products, so why would we need to add any energy to do that? But if there were no barrier to that last step, then the intermediates would not exist at all. As soon as they started to form, they would immediately slide down the hill and become products, That's kind of what happens in the other, concerted mechanism. What causes this barrier? That's less obvious, but it can involve factors such as organizational barriers. Maybe the nucleophile must approach the cation from a specific direction, and maybe the nucleophile itself has to be turned a specific way to bond with the cation. Organizing these things requires a small amount to energy. There may also be issues of steric crowding as the nucleophile and cation are brought closer together.
In the one we labeled Mechanism C, there was direct displacement of the leaving group by a nucelophile:
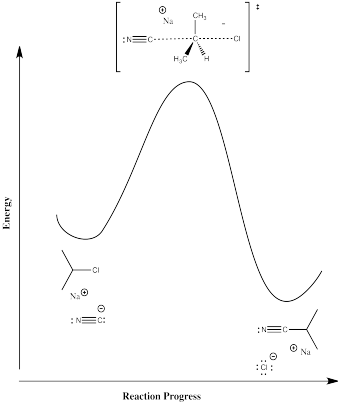
Figure NS2.6. Reaction progress diagram for a 1-step reaction.
Now, there are no intermediates at all. The nucleophile just comes in and pushes the halide off, takings it place on the carbon. The barrier involves all of the factors of the two-step mechanism at once. There is some bond-breaking requiring an initial input of energy, although this time we start to get some bond-making right away, which releases some energy. There are also the same organizational and steric considerations we saw in the second step of the previous mechanism.
Problem NS2.2.
Compare mechanism B and C in terms of your expectations of the following parameters:
a) Activation enthalpy.
b) Activation entropy.
There isn't necessarily a reason to believe that mechanism B is the correct mechanism and mechanism C is the wrong one, or vice versa. Either one may be possible. You may need to do some work in order to figure out which one really happens. Some experiments may help to highlight what is going on.
Problem NS2.3.
If charged intermediates are suspected along a reaction pathway, insight can sometimes be gained by running a reaction in a more polar solvent and comparing its rate to that of the reaction in a less polar solvent.
a) Are charged intermediates present, either in mechanism B or C?
b) Explain how each of these mechanisms might behave in a more polar solvent.
Problem NS2.4.
Sometimes, a distinction between two possible mechanisms can be gained by comparing rate laws expected from each mechanism.
a) What do you think is the likely rate-determing step in mechanism B?
b) What do you expect will be the rate law for mechanism B?
c) What do you think is the likely rate-determing step in mechanism C?
d) What do you expect will be the rate law for mechanism C?
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: