Reactivity in Chemistry
Aliphatic Nucleophilic Substitution
NS8. Nucleophilicity
The nucleophile can sometimes play a pronounced role in nucleophilic substitutions. It seems reasonable that some nucleophiles might be better than others, and so maybe those nucleophiles are more likely to undergo a nucleophilic substitution reaction. If we measure rates of substitution reactions involving halide nucleophiles in a aprotic solvent such as dimethylsulfoxide (DMSO), (CH3)2SO, we observe this trend:
fastest = F- > Cl- > Br- > I- = slowest
This trend may not be surprising, because it mirrors what we know about basicity. A base is an electron pair donor. A nucleophile is also an electron pair donor, although this term usually refers specifically to donating an electron pair to a carbon electrophile. We know from Bronsted acid-base chemistry that fluoride is the strongest base among this group, whereas iodide is the weakest. That's most often understood through consideration of anion stability. An iodide anion is quite polarizable and consequently it is quite stable compared to a fluoride anion. Remember, polarizability is a more important factor than electronegativity when comparing anions within a column of the periodic table because the sizes of the anions vary dramatically. So the same factor that makes fluoride more reactive as a base also makes it more reactive as a nucleophile.
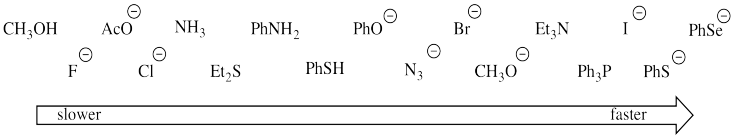
Nucleophilicities can vary with solvent, however. In particular, some nucleophiles that are the fastest-acting in aprotic solvents are actually the slowest-acting in protic solvents. The halides that we saw before are one set of examples, but there are others, too. The following relative rates have been observed when a variety of nucleophiles reacted with methyl bromide in methanol:

Figure NS8.1. Trends in nucleophicity in alcohol solvent. Note: Ph = phenyl, C6H5; Ac = acetyl, CH3C=O; Et = ethyl, CH3CH2.
Problem NS8.1.
Sometimes we can draw general conclusions about kinetic factors by looking at sub-groups among the data. Determine how the following factors influence nucleophilicity (the ability of a species to act as a nucleophile). Support your ideas with groups of examples from the data (preferably more than just a pair of entries).
a) charge on the nuclophile
b) size of the atom bearing the lone pair
c) electronegativity of the atom bearing the lone pair
d) delocalization of charge
Some of the species react much more quickly with methyl bromide because they are better nucleophiles than others. But why have some of these nucleophiles apparently slowed down in methanol compared to when they were in dimethylsulfoxide?
We already had the idea that anion stability may play a role in the rate of a nucleophilic reaction, just as it plays a role in base strength. The more stable an anion is, the less reactive it is, and the more slowly it will undergo reaction. So what if there is an additional factor in methanol solvent that stabilizes some of the nucleophiles, making them less likely to react? How could protic solvents stabilize some of these nucelophiles?
The answer is not exactly "hydrogen bonding", but it is a related idea. Small anions such as fluoride can definitely hydrogen bond with the solvent, and we could understand if that strong interaction stabilizes the fluoride and holds it back from reacting with the electrophile. It's believed that similar, weaker interactions form between the partially positive OH proton of methanol and the other halides. Chloride anion doesn't hydrogen bond, which really just means that the ion-dipole interaction between the methanol and chloride falls a little below that very strong threshold that we find with fluoride. Nevertheless, the interaction between methanol and chloride seems to be a little stronger than the interaction between methanol and bromide, and the weakest interaction is between methanol and iodide. It's the solvent interaction that reverses the nucleophilicity of the halides in methanol. The same thing happens in other alcohols or in water.
The strength of this interaction with protic solvents depends on hard and soft acid base factors. This is a concept that pops up here and there in organic and inorganic chemistry. Hard and soft acid base theory is rigorously understood using molecular orbital theory, but there is a simple shorthand that you can use as a guide. Just remember that hard acids interact strongly with hard bases and soft acids interact strongly with soft bases. But what is a hard base? A soft base? Hard bases are things like fluorine, oxygen, and nitrogen. Those are the same atoms that can hydrogen bond (you might remember the mnemonic from biology class, "hydrogen bonding is FON"). What do they hydrogen bond to? Hydrogen. H+ is a hard acid, along with the alkali metal ions in its column of the periodic table, and the alkaline earths in the next column: things like Li+ and Mg2+. These are all small ions in which the charge is very concentrated. Larger, more polarizable ions like gold and mercury, Au+ and Hg+ or sulfide and selenide, S2- and Se2- are soft.
The other factors that we learned about in acid-base chemistry work the same way regardless of solvent. A negatively-charged solvent is more nucleophilic than one without a charge. Within a row of the periodic table, a more electronegative anion is more stable and less nucleophilic than a less electronegative one. If the negative charge is delocalized by resonance, the nucleophile becomes less nucleophilic than it would be without resonance. The polarizability factor, which works within a column of the periodic table, is the only one that reverses depending on solvent.
Problem NS8.2.
Nucleophilicity plays a strong role in the rate of one type of substitution mechanism (SN1 vs SN2), but not the other.
a) In which mechanism is it important? Support your idea.
b) Is the reaction of methyl bromide likely to proceed via this mechanism? Why or why not?
Problem NS8.3.
A trend very similar to the data in Figure NS8.1. is found in substitution reactions of py2PtCl2 (py = pyridine) in methanol. Draw a mechanism for this substitution and explain why nucleophilicity plays an important role.
Problem NS8.4.
Very fast nucleophiles are sometimes more likely to undergo SN2 reactions than SN1 reactions. Explain why.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: