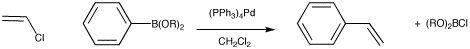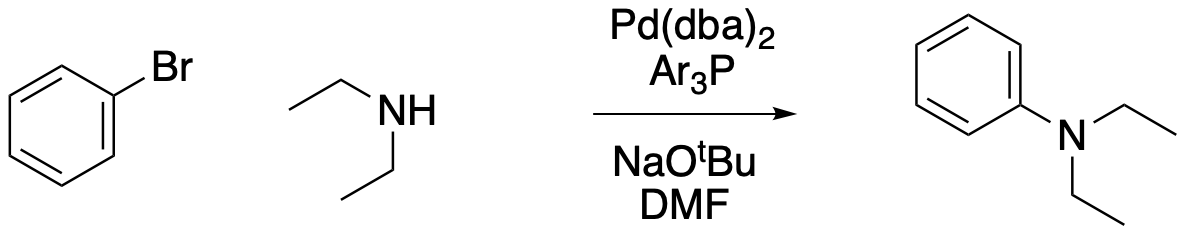Oxidative Addition & Reductive Elimination
OA.6. Palladium Coupling Reactions in Organic Synthesis
Oxidative addition and reductive elimination are key steps in industrial catalysis. For example, both steps are featured in palladium-catalyzed cross-coupling reactions, the subject of the 2010 Nobel Prize in Chemistry. The prize was awarded to Richard Heck of the University of Delaware, Ei-Ichi Negishi of Purdue University and Akira Suzuki of Hokkaido University. With these reactions, workers in a variety of fields can make molecules that otherwise would be quite difficult to make. These molecules in turn may be important pharmaceuticals or useful compounds for electronic displays in computers and other devices, to name just a couple.
The Negishi reaction involves catalytic addition of an organozinc nucleophiles to vinyl halides. Remember, alkyllithium and alkylmagnesium compounds are nucleophilic at carbon because the carbon-metal bond is quite polar. The partially negative carbon acts as the source of electron density in the reaction. Alkylzinc compounds work the same way as alkyllithium and alkylmagnesium compounds. In fact, there is an almost identical reaction, called the Kumada reaction, that uses organomagnesium compounds as nucleophiles in palladium-catalyzed cross couplings. Negishi coupling also works with aryl substrates, such as bromobenzene. The zinc portion can also be aromatic or a vinyl group.
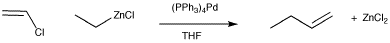
Figure OA6.1. The Negishi cross-coupling reaction.
At first glance, this looks like a straightforward nucleophilic substitution reaction, but the reaction actually follows a different pathway. The catalytic cycle believed to operate for this reaction involves the crucial oxidative addition of the vinyl halide (CH2=CHX) to the metal. The alkylzinc delivers the alkyl nucleophile to the metal via more conventional nucleophilic substitution at palladium. Once both pieces are both on the metal, they can reductively eliminate together. The oxidative addition is important because a nucleophilic substitution at carbon (SN2) wouldn't be possible with this vinyl halide. SN2 reactions happen at sp3 carbons, not at sp2 carbons. The oxidative addition accomplishes part of this coupling by breaking the C-Cl bond in one step, resulting in both the chloride and the vinyl attached to a Pd(II). This oxidative addition step works even better with the larger halogens, bromine and iodine, and also with sulfonates, especially triflate (OSO2CF3). The coupling is later finished by bringing the two hydrocarbon groups together in the reductive elimination step, a step that also restores the palladium to its initial oxidation state, Pd(0), turning the metal over so it can begin another catalytic cycle.
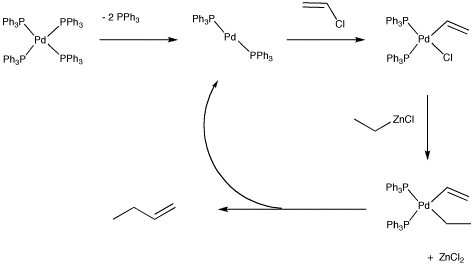
Figure OA6.2. Catalytic cycle for the Negishi cross-coupling reaction.
That trans-metallation step is just the transfer of an alkyl, vinyl or aryl ligand from one metal or metalloid to another. There is some evidence that in these cases the transfer goes through a bridging halide intermediate. That is, the halide on the metal coordinates to the alkylating agent before the ligand is transfered.
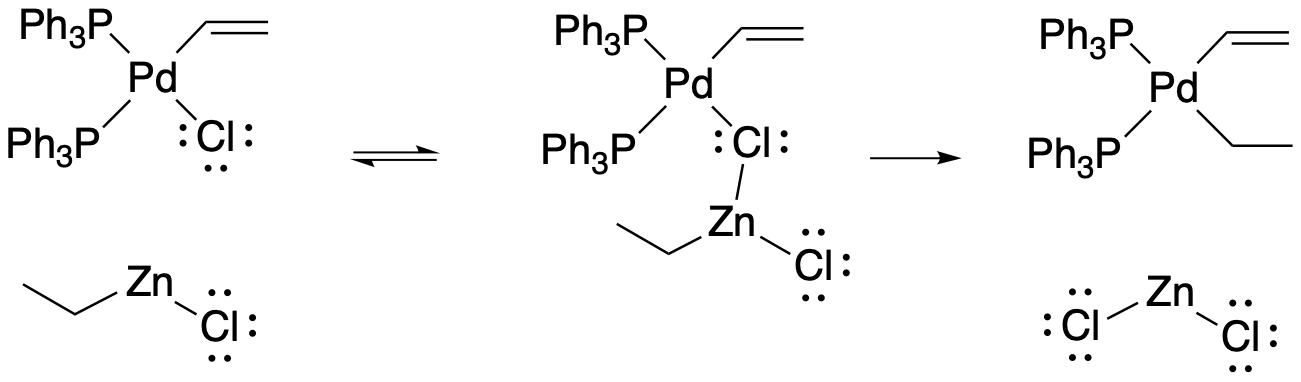
Figure OA6.3. Trans-metalation through a bridging halide intermediate.
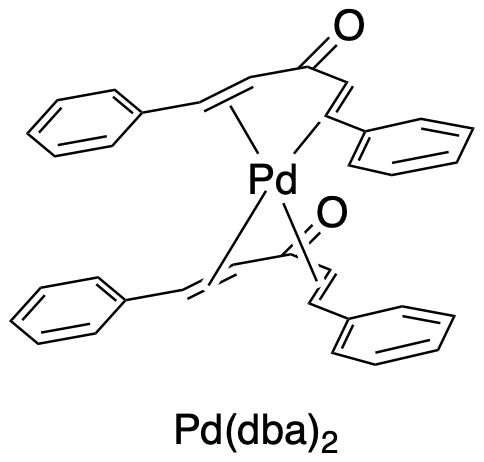
There are lots of different catalysts used for reactions such as the Negishi coupling. Nickel catalysts are pretty common, but we're going to focus on palladium catalysis because of the wider range of similar reactions that use palladium. Several palladium compounds are commonly used. These include simple salts like PdCl2 and Pd(OAC)2 as well as Pd(0) complexes like Pd(PPh3)4. Another common Pd(0) source is Pd(dba)2 and the related form, Pd2(dba)3. These are alkene complexes; Pd(dba)2 is shown below.
Figure OA6.3. A palladium complex used as a coupling catalyst precursor.
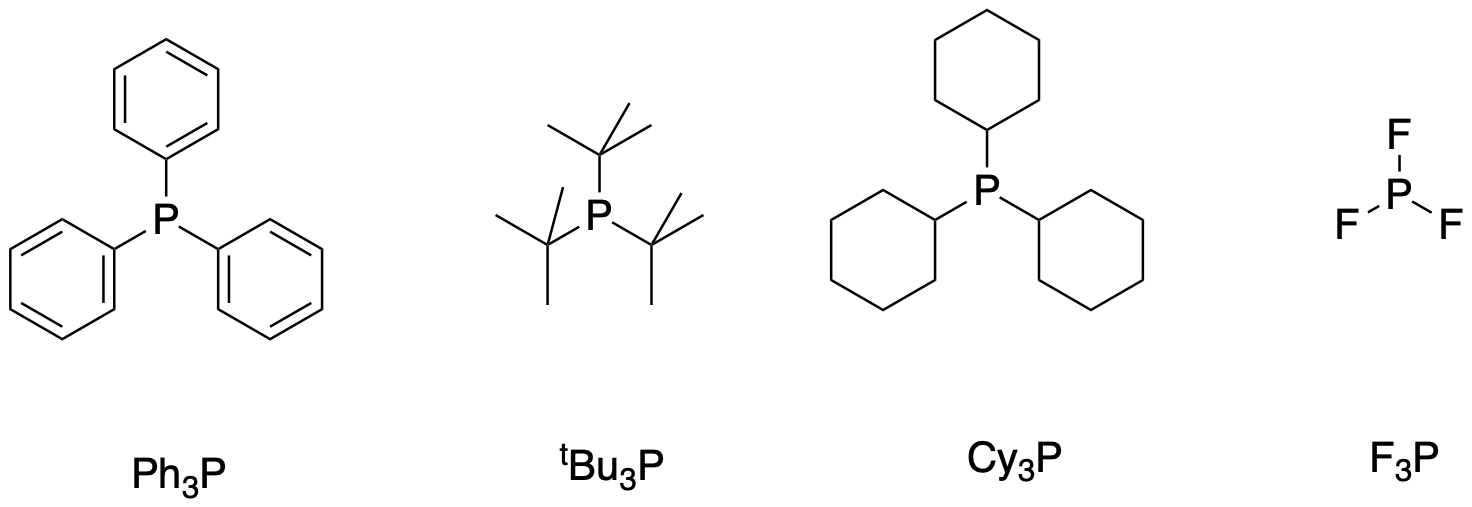
In addition, these kinds of reactions generally use phosphine promoters. Phosphines and related compounds accelerate these reactions by acting as ligands on the metal. They can help make the metal atom or ion more soluble, but they also tune the reactivity of the metal by how strongly they donate electron density (or even subtly withdraw electron density). A few representative phosphines are shown below.
Figure OA6.4. Some examples of phosphines used as promoters in coupling reactions.
Problem OA6.1.
Label each step of the catalytic cycle for the Negishi reaction with the appropriate term (oxidative addition, etc).
There are many other examples of coupling reactions in organic synthesis. The Suzuki reaction is somewhat similar to the Negishi reaction. Instead of a zinc compound providing the nucleophile, this role is played by an organoboron compound. Just like organolithium, organomagnesium compounds, and organolithium compounds are nucleophilic at carbon because of the polarity of the carbon-metal bond. Boron is considered a metalloid and the boron-carbon bond is also polarized with a partial positive charge on the boron and a partially negative charge on the carbon.
Figure OA6.5. The Suzuki reaction.
Both the Negishi and the Suzuki reactions involve Pd(0) catalysts. This low-oxidation state catalyst makes it easier for oxidative addition to occur. Nevertheless, Pd(II) salts are sometimes used in palladium coupling reactions. In these cases, it is believed that the reaction conditions result in reduction of the palladium from Pd(II) to Pd(0). In other words, some other compound in the system donates individual electrons to the palladium to return it to Pd(0). For example, phosphines are normally added to these reactions to act as ligands; these phosphines are typically present in an equilibrium between coordinated phosphine and free phosphine. Phosphine is also capable of acting as a reducing agent, transfering electrons to a metal ion to lower the metal oxidation state. Thus, the metal complex shown in the reaction conditions is often a pre-catalyst, meaning it may be convenient to add this particular complex to the reaction, but once in there it gets turned into a different form that actually does the work.
Problem OA6.2.
By analogy with the Negishi reaction, draw a catalytic cycle for the Suzuki reaction.
Stille coupling is just one more variation, very much like the Negishi and Suzuki reactions. This time, the nucleophilic component comes from an organotin compound. Once again, organotin compounds are nucleophilic because of a polar carbon-tin bond. Carbon is more electronegative than tin, so consequently the carbon is partially negative and nucleophilic. That's just what we have learned about carbon-lithium, -magnesium, -zinc, and -boron bonds. The Stille reaction typically uses an aryltributyltin or vinyltributyltin compound such as PhSnBu3 or CH2=CHSnBu3. Like the other reactions, it couples that nucleophilic piece with an aryl halide or vinyl halide.
Figure OA6.6. The Stille reaction.
Problem OA6.3.
By analogy with the Negishi reaction, draw a catalytic cycle for the Stille reaction.
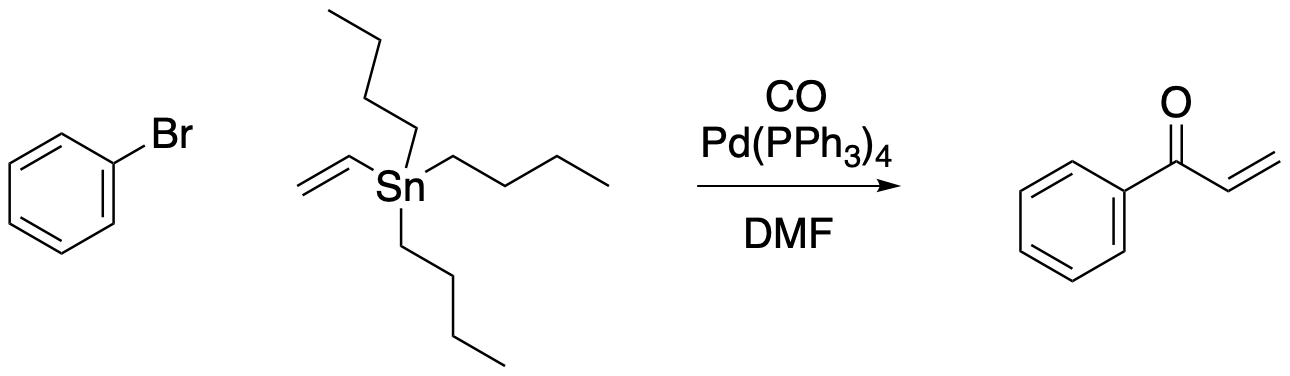
There is a variation of a Stille coupling, called a carbonylative Stille coupling. It's the same reaction, run under an atmosphere that contains carbon monoxide. Under these conditions, a unit of CO is incorporated into the structure between the two fragments that are being coupled together. The result is a new ketone.
Figure OA6.7. The carbonylative version of the Stille reaction.
Problem OA6.4.
By analogy with the Negishi reaction, draw a catalytic cycle for the carbonylative Stille reaction.
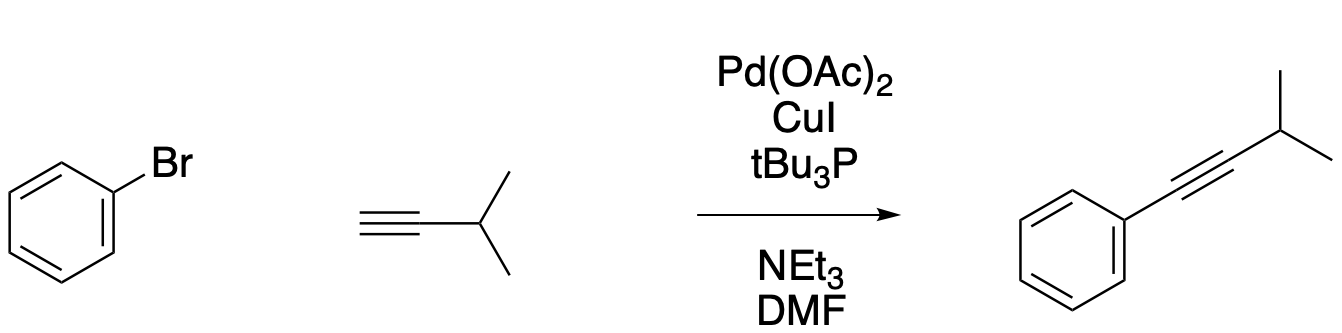
All of these palladium coupling reactions use aryl or vinyl halides. They may not be strictly electrophiles in the sense of nucleophilic substitution reactions, but they do bring electrophiles to mind, so it is easy to think of these reactions are requiring an electrophilic partner and a nucleophilic partner in order to couple together. The Sonogashira reaction, like the others, involves oxidative addition of a vinyl halide or an aryl halide. However, it doesn't involve addition of a reagent containing a nucleophilic C-M bond such as C-Sn, C-B, or C-Zn. The catalytic cycle is somewhat different because a copper(I) salt and a mild base are used to deprotonate an alkyne and generate an alkynide ion, which is then transferred to the palladium. Thus, the overall reaction is a coupling between an aryl or vinyl halide and a terminal alkyne.
Figure OA6.8. The Sonogashira reaction.
Problem OA6.5.
Based on the description, draw a catalytic cycle for the Sonogashira reaction.
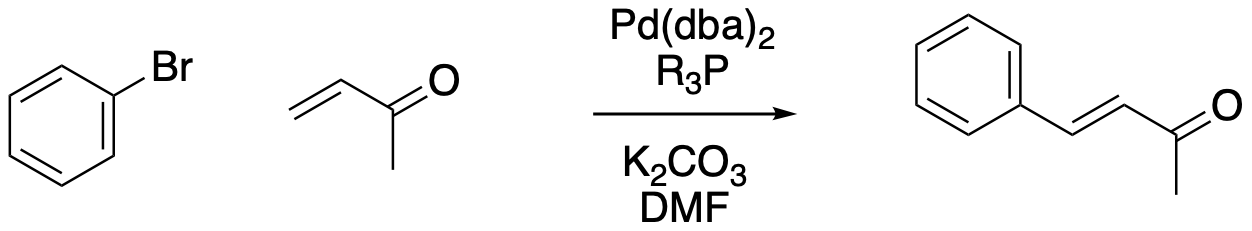
The Heck reaction is the last example we'll look at here. It is like the Sonogashira reaction in pairing a vinyl or aryl halide bond cleavage with a C-H bond cleavage, but whereas Sonogashira uses a terminal alkyne, the Heck reaction involves activation of a vinylic C-H bond. In most cases, the alkenes involved in this reaction are α, β-unsaturated carbonyl systems like enones. That's a pretty significant difference from the other cases, because we often think of enones as electrophilic, not nucleophilic. In this case, the C=C bond really does act as a nucleophile, coordinating to the palladium. Instead of the two fragments coupling together via reductive elimination, they are brought together through a 1,2-insertion step. The vinyl group is restored in a subsequent β-hydride elimination.
Figure OA6.9. The Heck reaction.
Problem OA6.6.
Based on the description, draw a catalytic cycle for the Heck reaction.
That's a lot of similar reactions to accomplish similar tasks. If you need to recall which name goes with which reaction, here is a mnemonic device that may help.
Suzuki makes bikes. B is for boron. Stille is for stannum, the latin word for tin (and the reason the symbol for tin is Sn). Negishi uses a more familiar nucleophile. Heck replaces an H from an alkene.
Problem OA6.7.
Buchwald-Hartwig coupling has its origins in a catalytic reaction developed in the early 20th century by Irma Goldberg and later improved by both the Buchwald (MIT) and Hartwig (UC Berkeley)labs. Related reactions are sometimes called the Ullman-Goldberg-Buchwald reaction in reference to these historic roots.
Draw a catalytic cycle for this reaction.
Problem OA6.8.
Fill in the blanks in this multi-step synthesis involving a palladium coupling reaction.
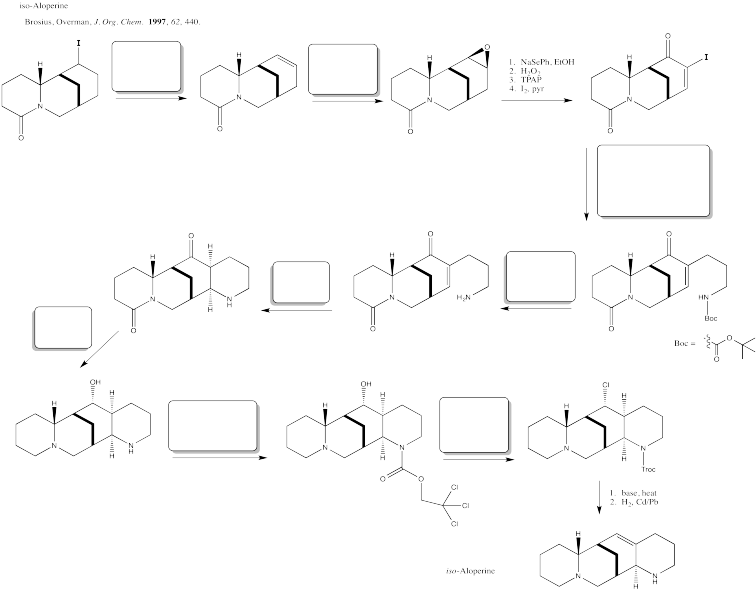
Problem OA6.9.
Fill in the blanks in this multi-step synthesis involving a palladium coupling reaction.
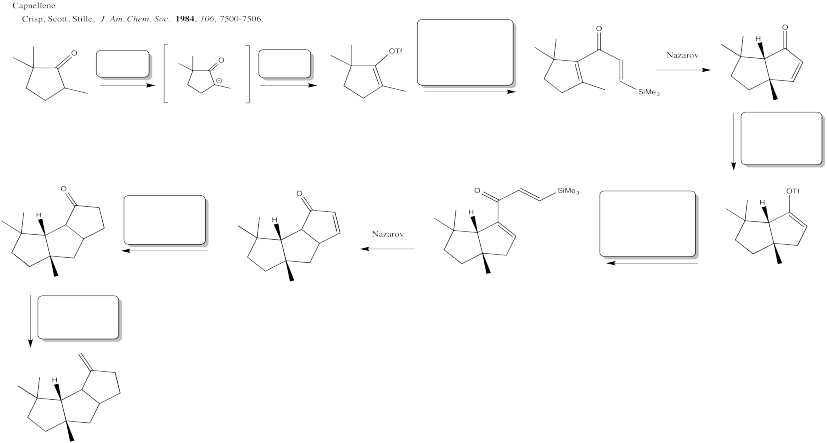
Problem OA6.10.
Fill in the blanks in this multi-step synthesis involving a palladium coupling reaction.
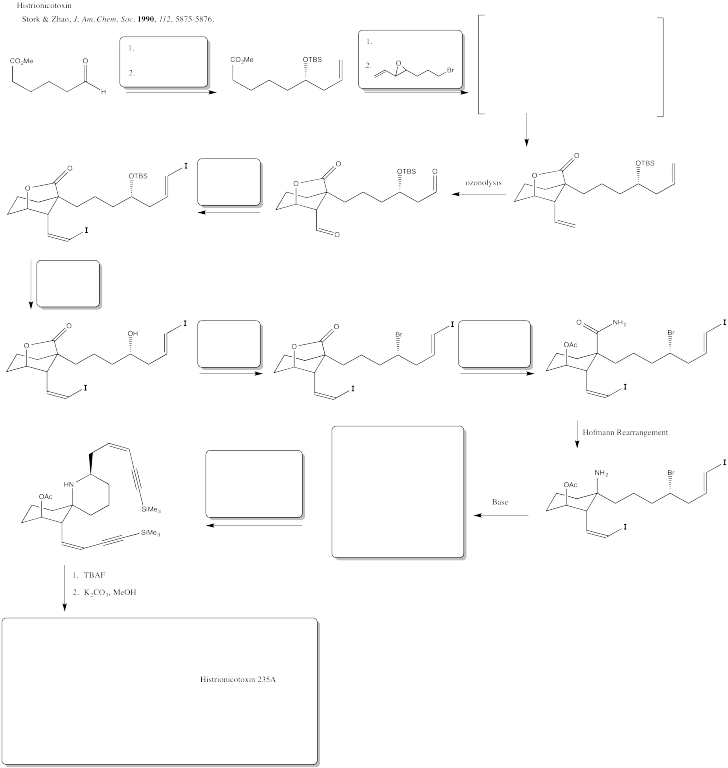
Read more about these Nobel Prize-winning reactions at the Nobel Prize website.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Oxidative Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry