
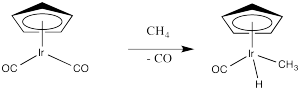
Figure OA4.1.
Reactivity in Chemistry
Oxidative Addition & Reductive Elimination
OA.4. Concerted Oxidative Addition
Concerted oxidative addition is a more general reaction than polar addition, in the sense that it is not restricted to compounds that can undergo aliphatic nucleophilic substitution. It could also be thought of as non-polar oxidative addition, because it does not involve charged intermediates as seen in the polar mechanism.
Aryl halides, for example, do not undergo nucleophilic substitution, but they do undergo concerted oxidative addition.
Instead of proceeding step by step, the addition of both fragments is synchronized. They add to the metal at the same time.
Figure OA4.1.
At first, it's difficult to understand this mechanism in terms of nucleophiles and electrophiles. The reaction is generally explained in terms of molecular orbital interactions, however, that can be thought of as nucleophile-electrophile interactions.
There are interactions involved in a concerted or non-polar oxidative addition.
There is sigma bond donation from a bonding orbital in the substrate into a metal p orbital. This interaction is shown on the left of figure OA4.2.
There is donation from a metal d orbital into an antibonding orbital on the substrate. This interaction is shown in the middle of figure OA4.2.
Overall, a pair of electrons are donated from the substrate to the metal, and a pair of electrons are donated from the metal to the substrate.
Figure OA4.2. Molecular orbital interactions in a non-polar oxidative addition.
Figure OA4.3. A curved arrow representation of non-polar oxidative addition.
Problem OA4.1.
Provide a mechanism, with curved arrows, for the following reaction.
Problem OA4.2.
Propose a reason for the fact that one of the following dimethyl palladium compounds undergoes reductive elimination, but the other one does not.
Problem OA4.3.
Frequently, oxidative addition and reductive elimination are combined with other reactions into catalytic cycles. These cycles form the basis of important processes used to make valuable materials. Propose catalytic cycles for the following reactions. You don't need to draw curved arrows; just provide the intermediate formed after each reaction step.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Oxidative Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry