Reactivity in Chemistry
Photochemical Reactions
PC7. Applications of Photochemistry: Photoredox Catalysis in Organic Chemistry
Photoredox catalysis is a powerful way to harness the power of light and use it to do reactions involving electron transfer. By promoting an electron to a higher energy orbital (usually through the absorbance of a photon), the electron becomes thermodynamically more driven to be donated to another reactant. Transfer of the electron to another orbital on another reactant becomes more feasible. The electron transfer may even switch from being uphill energetically to downhill energetically. That would switch the electron transfer from being one that is unlikely to occur to one that occurs easily. At the same time, the hole left behind where the electron used to be becomes a good place to transfer a new electron. Suddenly, there is space for an electron where there wasn't before, because the site used to be occupied. This site may now be able to accept an electron from another reactant. The photo-excited reactant becomes both a better reducing agent and a better oxidizing agent.

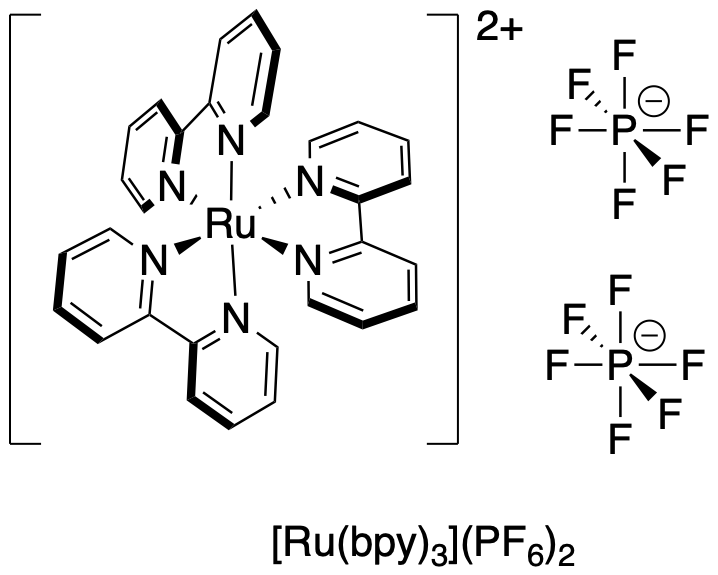
Figure PC7.1. Ru(bpy)3 ("roo-bippy") is a common photocatalyst.
[Ru(bpy)3]2+ complexes, such as [Ru(bpy)3][PF6]2, provide a good example of photo-active agents that take advantage of this chemistry. [Ru(ppy)3] is similar complex with similar properties that has been used in the same way. There are others, but these complexes have been used in a variety of applications and they are a good place to start. Excitement of [Ru(bpy)3]2+ can be accomplished easily using visible light sources, incluing LEDs. [Ru(bpy)3]2+[PF6]2 is a red complex, so it absorbs green light. The most prominent transition for this complex is a metal-ligand charge transfer. If we take that term literally, it means that when a photon is absorbed, we excite an electron from the metal to the ligand. [Ru(bpy)3]2+ contains a Ru2+ ion, so if we excite an electron from the metal to the ligand, we are left with a Ru3+ ion. At the same time, one of the neutral bipyridyl ligands becomes a bipyridyl anion.
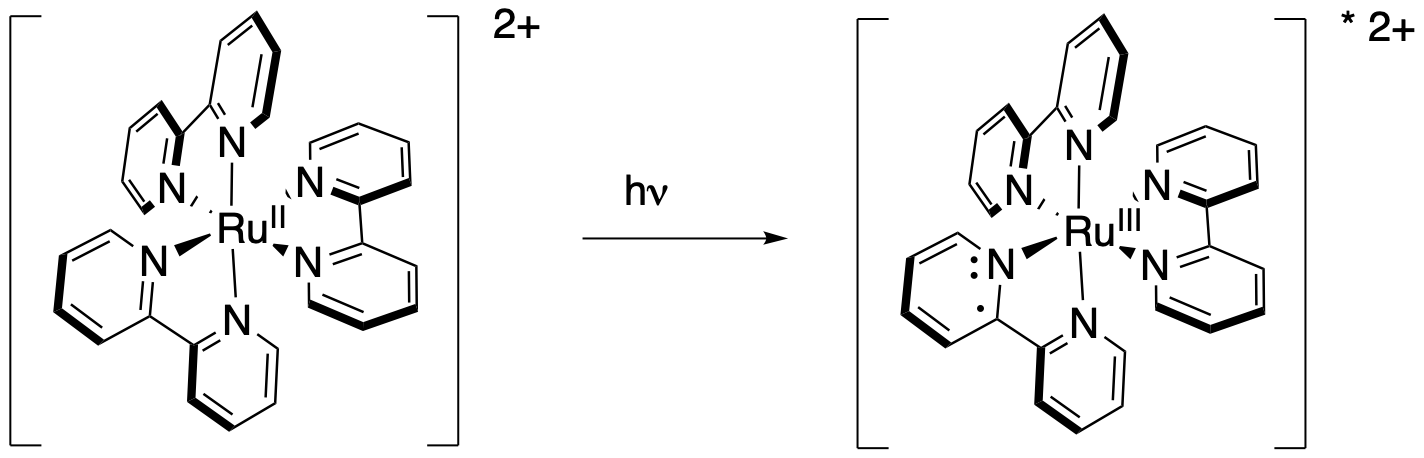
Figure PC7.2. Ru(bpy)3 has an MLCT band, so the excited electron is in the ligand whereas the hole is on the metal.
This change has significant consequences for redox chemistry. If we consider the oxidizing power of the metal ion, we have gone from an octahedral Ru2+ ion with an eg level that acts as the lowest occupied molecular orbital (LUMO). If the complex were to accept an electron, the electron would be placed in the eg level. By comparison, if photo-excitation leads to an octahedral Ru3+ ion, then there is a hole in the t2g level. That's a lower energy level than the eg. If an electron can be donated into a lower energy level, then the potential for that electron donation increases. At the same time, raising an electron into the π* orbital of the bipyridyl ligand makes the bipyridyl a strong reducing agent.
One of the reasons Ru(bpy)32+ is a popular choice for photocatalysis is its strong absorption of visible light. In addition, there are a lot of variations on this complex that are commercially available. Variety is important, because slight changes in the ligand environment can modulate the physical properties of these materials. That could include either the absorption maximum in the UV-visible spectrum or the redox potential of the photocatalyst. For example, although [Ru(bpy)3](PF6)2 and [Ru(bpz)3](PF6)2 look very similar, the excited state of [Ru(bpz)3](PF6)2 is a more powerful oxidizing agent by several hundred millivolts than the excited state of [Ru(bpy)3](PF6)2. These differences can be important in optimizing reaction conditions for new applications.
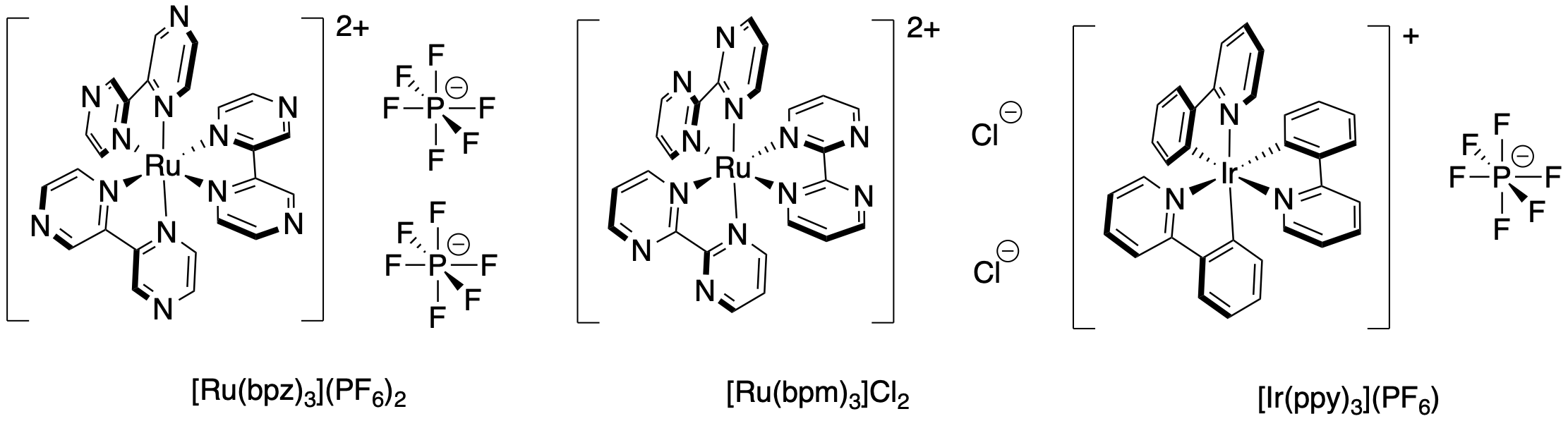
Figure PC7.3. There are variations on Ru(bpy)3 with different optical and electronic properties.
Problem PC7.1.
Propose a reason for the stronger oxidizing power of [Ru(bpz)3](PF6)2 compared to [Ru(bpy)3](PF6)2 .
To see how these advantages can be put to use, let's look at a specific application. This one was developed in the lab of Corey Stephenson at Boston University (now at Michigan). The application is fairly simple: how can we remove a halogen atom from a halogenated organic compound? These compounds are ubiquitous today, used in all kinds of applications from refrigerants to electronics, but they are associated with a range of environmental and health problems. People are motivated to find ways to break them down. One of the most reliable approaches in this case is to treat the halide with a tin hydride, replacing the halogen atom (chlorine in this case) with hydrogen. The only problem is that tin compounds are also toxic. That means one of the best ways to treat this one environmental problem only introduces another environmental problem. So, people don't actually do this, but it illustrates one reason why the Stephenson lab was doing something important by trying to find a catalytic method for halogen removal.
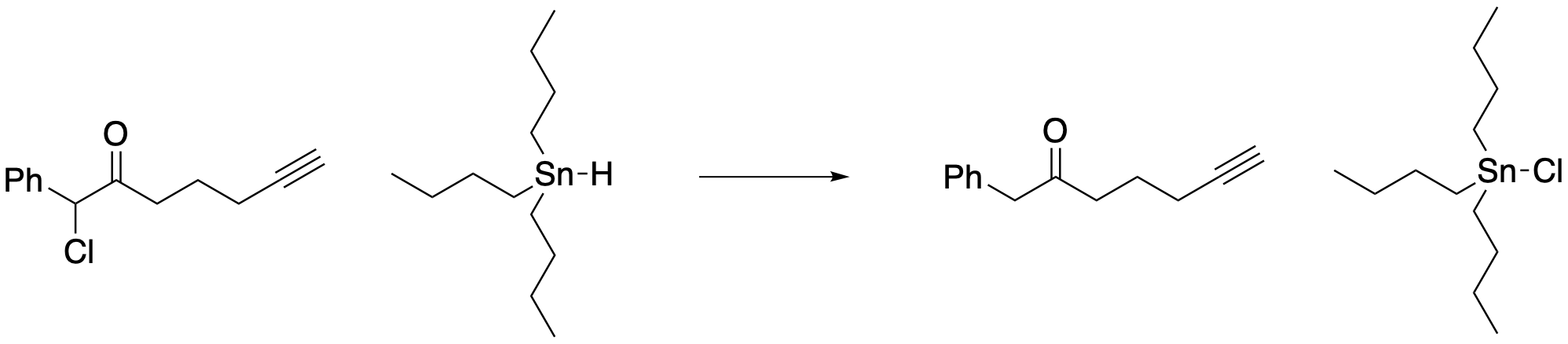
Figure PC7.4. The reduction of a halogenated organic compound using a tin reagent.
The Stephenson lab took an approach that used a catalytic amount of ruthenium complex and an amine as a sacrificial reductant (Jagan M. R. Narayanam, Joseph W. Tucker, and Corey R. J. Stephenson, J. Am. Chem. Soc. 2009, 131, 8756–8757). Catalytic amounts of materials are more efficient and more environmentally friendly because they can be continuously regenerated and re-used in the reaction. A "sacrificial" reductant is any compound that will be decomposed in some way in order to access its electrons; it can't be used catalytically because a stoichiometric amount of electrons is needed for the reaction to proceed. Usually an inexpensive, common material is chosen for this purpose.
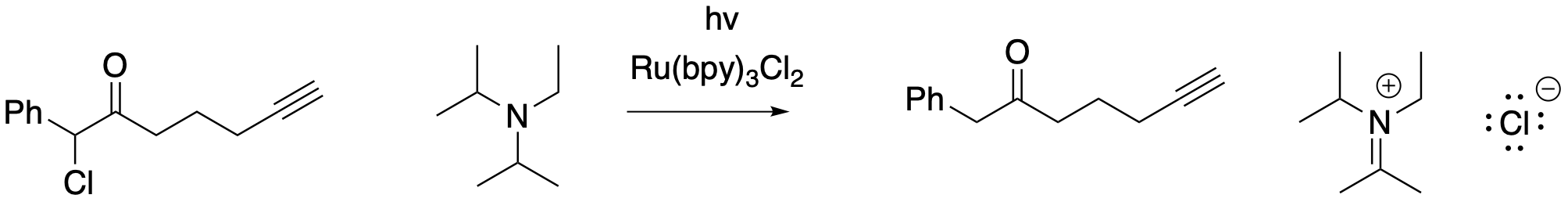
Figure PC7.5. Reducing the same halogenated organic compound with a photocatalyst.
Let's disassemble this reaction into its working parts. First of all, how is the halogen displaced in this case? Here, the chlorine is lost as chloride ion by reduction of the organohalogen compound. The reducing agent is that high-lying electron from the excited state of the catalyst, [RuIII(bpy)2(bpy.-)]. The organic substrate becomes a neutral radical.
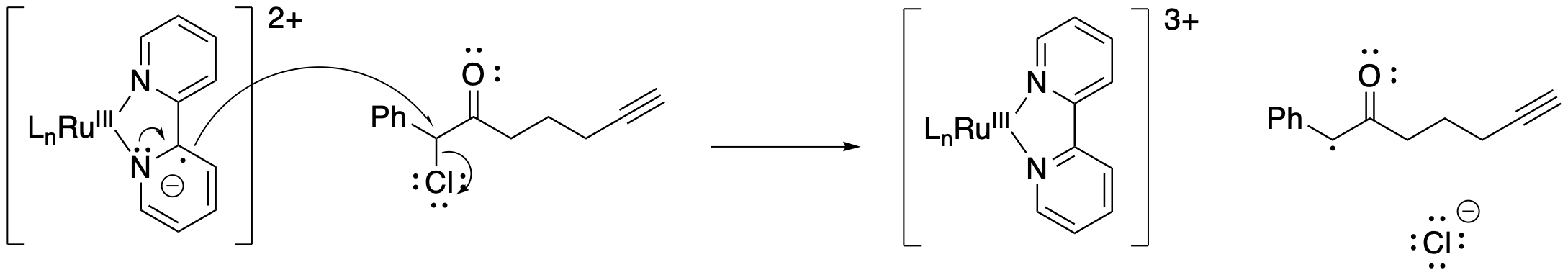
Figure PC7.6. The photocatalyst is an excellent reducing agent.
That leaves the photocatalyst in an oxidized state. To return to its original state and achieve catalytic turnover, it will need to be reduced again. That's what the sacrificial reductant is for. The amine donates one of its electrons to the ruthenium, returning the ruthenium back to its original Ru(II) state, which can then be photochemically excited to enter the cycle again. Amines are pretty good at doing this. Nitrogen and oxygen are two very common heteroatoms in organic molecules, but of the two of them, nitrogen has a much lower ionization potential, so it is much easier to strip the electron from a nitrogen than from an oxygen, which would otherwise be the obvious place to look for electrons. Putting it another way, although oxygen compounds are even more common than nitrogen compounds in nature, oxygen is more electronegative than nitrogen. Its electrons are at even lower energy than nitrogen's; consequently, it costs less energy to strip an electron from nitrogen than from oxygen.
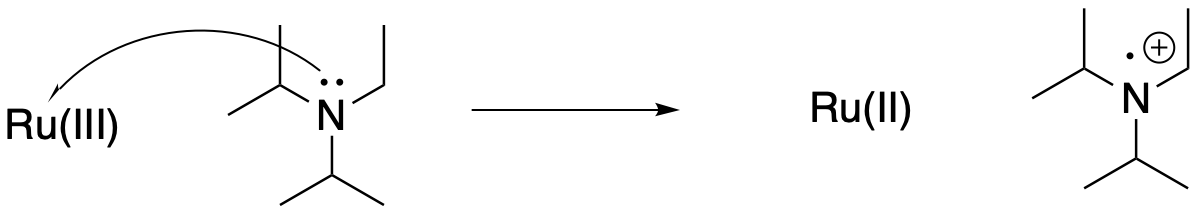
Figure PC7.7. The hole is filled using a sacrificial reductant.
The reaction isn't quite finished, yet. There is still a radical intermediate that needs a hydrogen atom. That missing piece is also supplied by the amine. The radical cation that is left after transferring an electron to the ruthenium easily yields a hydrogen atom, becoming an iminium ion. The iminium ion will decompose under aqueous conditions to become a ketone.
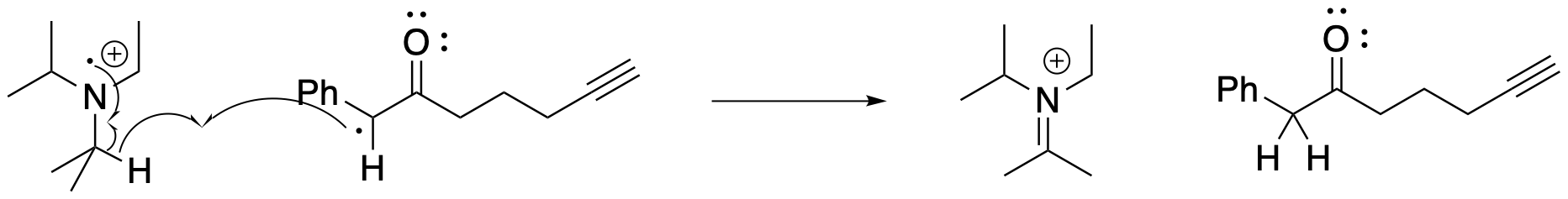
Figure PC7.8. A hydrogen atom is extracted from the oxidized amine.
Problem PC7.2.
Redraw the components of Stephenson's photocatalytic process as a catalytic cycle with Ru(bpy)3, using the other reactants to feed into the cycle.
The MacMillan lab at Princeton is another prominent research group involved in photoredox catalysis in organic chemistry. MacMillan shared the Nobel Prize in chemistry in 2021 for his work in catalysis, especially as it relates to the controlled production of specific enantiomers of chiral compounds. However, his research in photocatalysis has been equally important. An example of a reaction from this group is the following C-H bond activation and aromatic substitution.
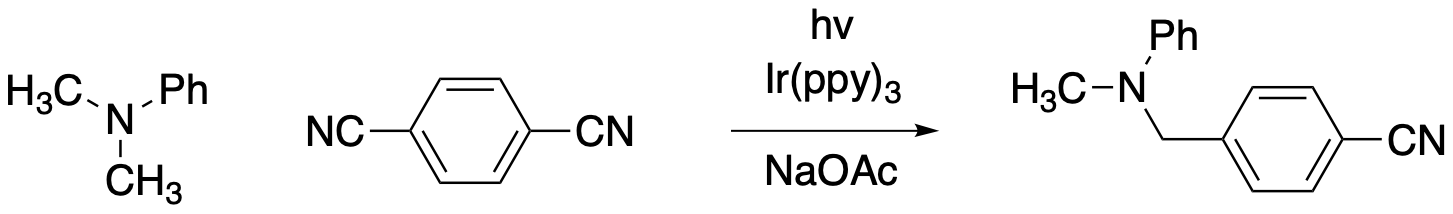
Figure PC7.9. Another example of photocatalysis.
The mechanism for this process centers on a catalytic cycle of the photocatalyst. The excited state iridium complex reduces the aromatic and oxidizes the amine. A radical coupling step ensues, followed by elimination of a cyanide leaving group.
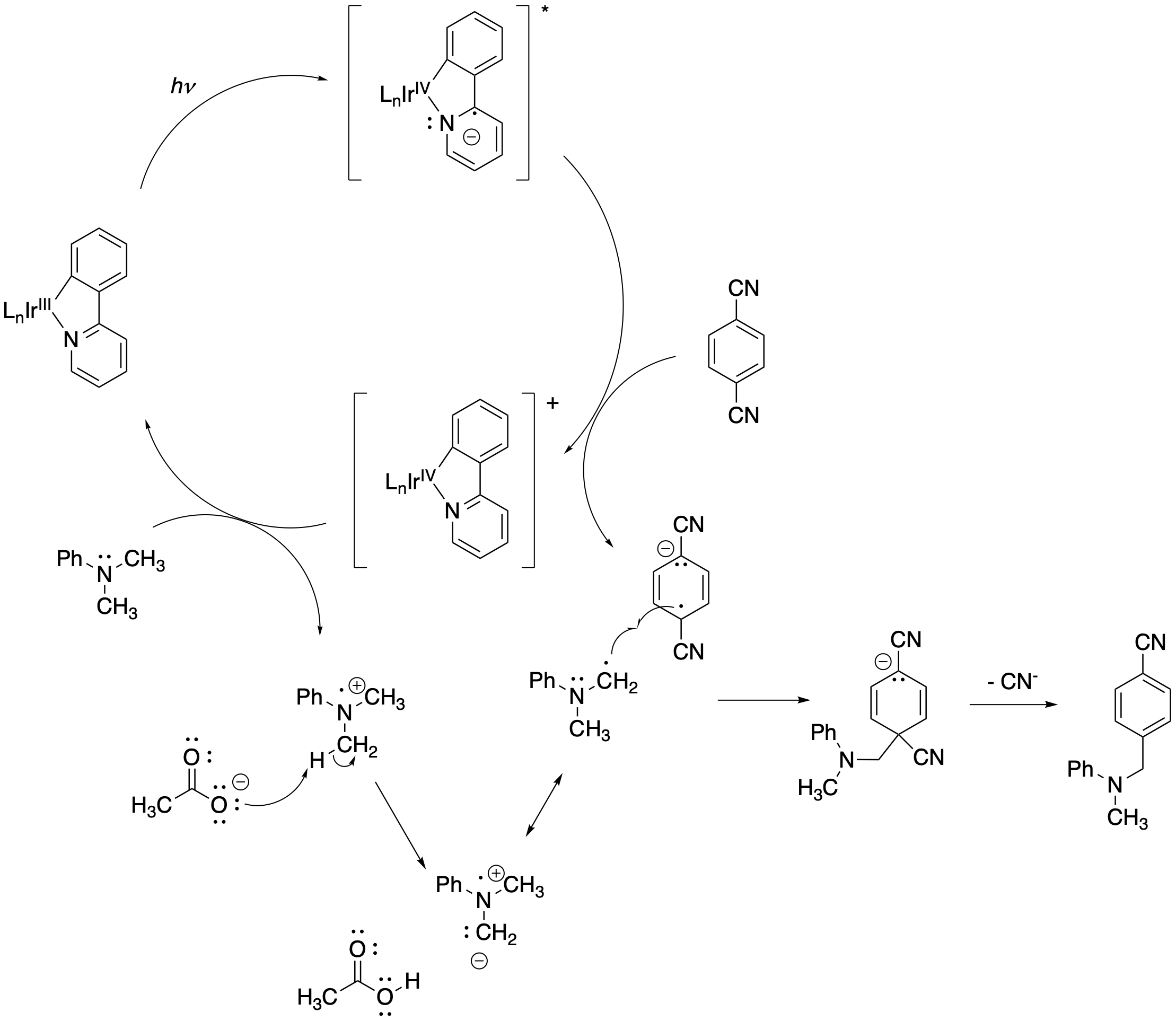
Figure PC7.10. The photocatalytic cycle for the example above.
Problem PC7.3.
a) Redraw the reaction of MacMillan's photocatalyst acting as a reducing agent.
b) Redraw the reaction of MacMillan's photocatalyst acting as an oxidizing agent.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: