Reactivity in Chemistry
Photochemical Reactions
PC4. Photolysis
We have seen that the absorption of photons (especially in the ultraviolet-visible spectrum) is connected to the excitation of electrons. After excitation, a number of different relaxation pathways lead back to the ground state. Sometimes, absorption of a photon leads to a vastly different outcome. Instead of just relaxing again, the molecules may undergo bond-breaking reactions, instead.
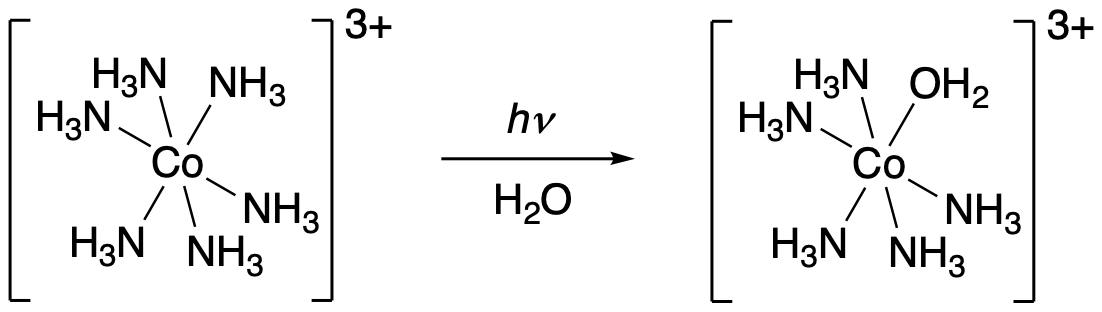
An example of this phenomenon is observed in the complex ion [Co(NH3)]63+. Addition of UV light to this complex results in loss of ammonia. In the absence of UV light, however, the complex ion is quite stable.

Figure PC4.1. Photochemical replacement of a ligand in an inert Co(III) complex.
In many cases, loss of a ligand is followed by replacement by a new one. For example, if an aqueous solution of [Co(NH3)]63+ is photolysed, an ammonia ligand is easily replaced by water.
Problem PC4.1.
Draw a d orbital splitting diagram for [Co(NH3)]63+. Explain why this complex is normally inert toward substitution.
Problem PC4.2.
Use the d orbital splitting diagram for [Co(NH3)]63+ to explain why this complex undergoes substitution upon irradiation with UV light.
Photolysis is the term used to describe the use of light to initiate bon-breaking events. Photolysis frequently involves the use of high-intensity ultraviolet lamps. The high intensity light is needed in order to provide enough photons to get higher conversion of reactant into a desired product.
In the case above, the Co(III) ion is inert to substitution because it is a low-spin d6 complex. All of its t2g orbitals are occupied, preventing new ligands from binding. Because its eg orbitals are all empty, it doesn't have the bond-weakening effect of occupied antibonding levels to loosen the hold of the old ligands. Nevertheless, ammonia isn't a very strong-field ligand. Can photons knock off stronger field ligands?
Yes, they can. Hexacarbonylchromium, Co(CO)6, is also a low-spin d6 complex. In this case, the metal isn't low-spin because of a high positive charge. It's low-spin because of strong field carbon monoxide ligands. Those ligands hang on tightly because of strong backbonding from the chromium. But one of them can also be replaced by a solvent molecule if subjected to strong ultraviolet-visible light. Once a CO ligand is photolyzed off, it is likely lost because CO is a gas; that means the equilibrium will shift to the right.
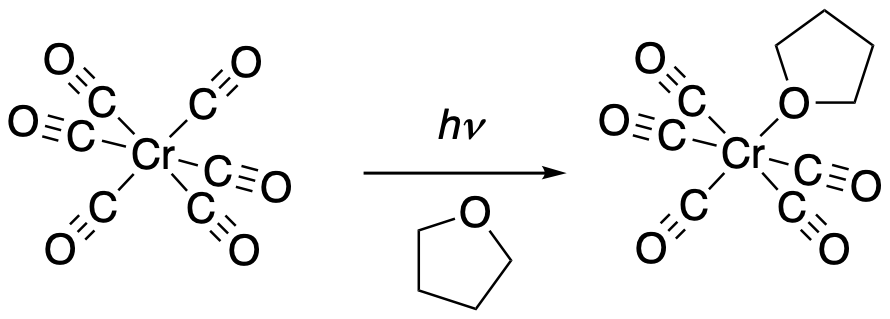
Figure PC4.2. Photochemical replacement of a ligand in a low-spin Cr d6 complex.
Replacement of a strong-field CO ligand by a weaker-field THF ligand can be useful. The THF complex can be stored and used later to easily introduce a variety of other ligands into the complex without having to bombard it with high energy photons.
There are lots of variations of this kind of reaction. Up to three CO ligands can eventually be replaced. They might be replaced by three individual ligands, or they could be replace by one tridentate ligand, such as an aromatic ring. Chelation makes that tridentate ligand hold on more tightly. It's not that likely that all of the carbonyl ligands would be so easily replaced, however, because as each one leaves, there is more and more electron density on the chromium available for backbonding. As each Co is knocked off, the others bind more and more tightly.
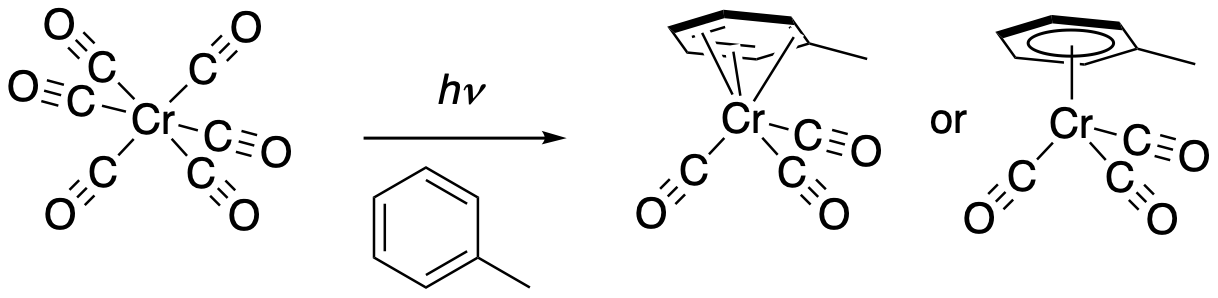
Figure PC4.3. Photochemical replacement of 3 ligands in a low-spin Cr d6 complex.
Two very different things could happen as a result of photon absorption. In one case, the molecule absorbs the photon, then somehow relaxes again, remaining unchanged overall. In the other case, the absorption of the photon results in bond cleavage and the formation of a new product. As a result, for every photon absorbed, there is a certain chance that the molecule will actually undergo a reaction, and a certain chance that the molecule will just relax again.
"Quantum yield" is an expression used to define the efficiency of a photolytic reaction. The quantum yield is just the number of molecules of reactant formed per photon absorbed. On a macroscopic level, we might say it is the number of moles of reactant formed per mole of photons absorbed.
Quantum yield = number of molecules of product formed / number of photons absorbed
The higher the quantum yield, the more efficient the reaction, because it requires less light in order to successfully form the product.
Problem PC4.3.
Calculate the quantum yield in the following cases.
a) 6 mmoles of product results from absorption of 24 mmoles of photons.
b) 54 mmoles of photons are required to produce 3 mmoles of product.
c) 1.2 x 10-6 moles of product are formed after absorption of 4.2 x 10-5 moles of photons.
In practice, determination of quantum yield is complicated because of the need to calculate exactly how many photons have been absorbed, in addition to how much product has been formed.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: