Reactivity in Chemistry
Radical Reactions
RR9. Radical Polymerization
Radical addition to alkenes can be applied to the production of macromolecules. Like other polymerization reactions involving alkenes, it involves the formation of a reactive intermediate by the action of an initiator on an alkene. A chain reaction results in which other alkenes are enchained into a polymer.
The term "initiator" here is used in a slightly different way than we have used it with other radical reactions. The radical initiator has already undergone its reaction to form a radical. That radical then initiates chain growth. This step is really a propagation step in terms of types of radical elementary reactions, because one radical leads to a new radical.
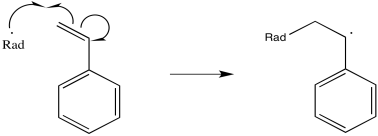
Polystyrene is one example of a material that is frequently prepared via radical conditions. During the reaction, a radical adds to the double bond of the alkene.

Figure RR9.1. Initiation of polymerization by a radical. The monomer turns into a growing chain.
The newly-formed radical, in regular alkene addition, would then react with something to abstract an atom and achieve stable, closed-shell configuration. However, in a polymerization reaction, alkene molecules are intentionally packed closely together. Either they are very concentrated in solution or else they are neat (with no solvent at all). As a result, the newly-formed radical just gobbles up another alkene.
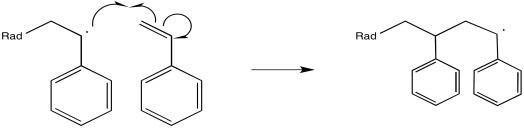
Figure RR9.2. Propagation of a growing radical chain.
There are lots of ways to carry out this reaction. One way would be to take some styrene, heat it up until it melts, and add some benzoyl peroxide.
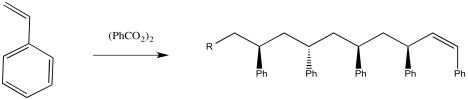
Figure RR9.3. Radical polymerization of styrene initiated by benzoyl peroxide (BPO).
Radical polymerization is a critical method of preparing polymers because it can be used in such a broad range of cases. The figure below shows a number of monomers that are commonly polymerized under radical conditions.
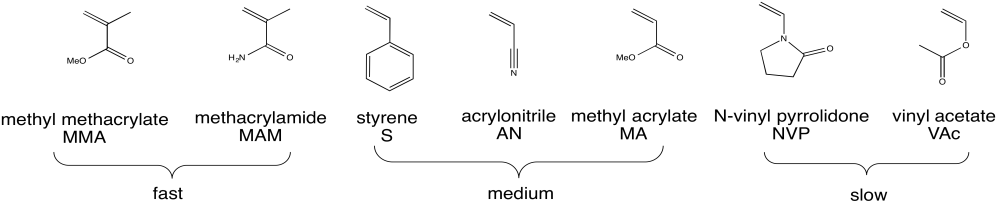
Figure RR9.4. A wide variety of monomers that can be polymerized under radical conditions.
Problem RR9.1.
Look at the monomers listed above.
a) For each of the three groups (fast, medium and slow), identify what structural features the members of the group have in common that distinguishes them from the other groups.
b) Propose a reason why the fast group undergoes more rapid polymerization than the other groups.
c) Propose a reason why the medium group undergoes more rapid polymerization than the slow group.
Problem RR9.2.
Provide a mechanism for the polymerization of styrene in the presence of benzoyl peroxide, up through the first couple of propagation steps.
A radical polymerization, after the initiator gets going, is just a series of propagation steps in a row. How does it all stop? There must be a termination reaction. That can happen in a couple of different ways.
For example, two growing chains might encounter each other, head-to-head. A collision could bring the unpaired electrons together to form a new bond. Two growing polymer chains would come to an end at once. At the same time, the chain length and molecular weight doubles as two chains combine into one.
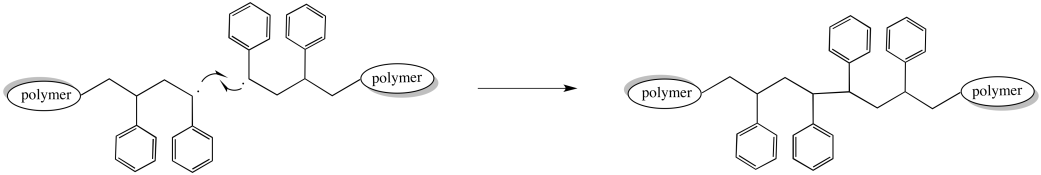
Figure RR9.5. A possible termination of growing radical chains through radical coupling.
Of course, most of the other chains in the polymerization continue growing normally. As a result, the distribution of molecular weights broadens dramatically. Some chains are twice as long as the others.
Alternatively, when two growing chains collide, one might carry out a hydrogen atom abstraction on the other. The hydrogen abstracted might come from the head of the chain, alpha to the active radical. That C-H bond is a little bit weaker, as bonds alpha to radicals typically are. The chain that picks up the hydrogen is no longer a radical. The chain that loses a hydrogen is no longer a radical, either. Both chains stop growing. Because other chains around them continue to grow, these one lag behind, resulting in a wider distribution of chain lengths.
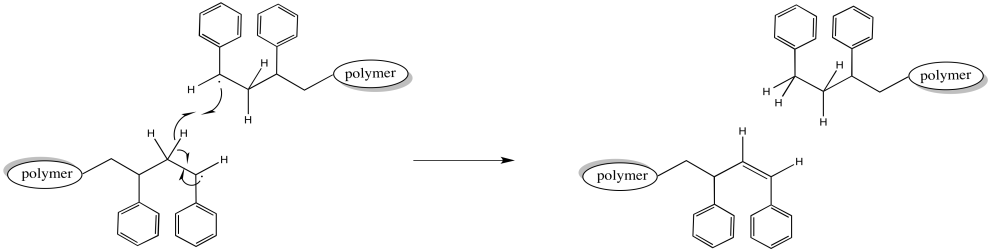
Figure RR9.6. A possible termination of growing radical chains through radical elimination.
Of course, a hydrogen atom abstraction could happen elsewhere along the chain. In that case, the chain that abstracted the hydrogen stops growing, as before. However, the chain that lost the hydrogen now has two radicals. That makes two sites of chain growth. This chain starts growing twice as fast as the others. Eventually, that's going to lead to a big difference in molecular weights.
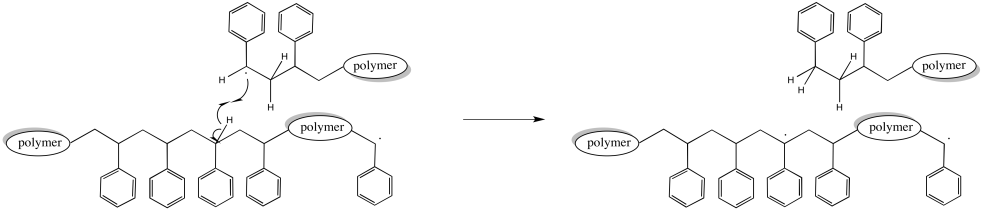
Figure RR9.7. A possible termination of a growing radical chain through hydrogen atom abstraction.
These termination events, all of which are possible and which occur more or less randomly, have profound consequences on the material produced.
There is an effect on chain length and molecular weight. Chains that have abstracted a hydrogen atoms come to a complete halt. They stop growing altogether. On the other hand, chains that have had a hydrogen stolen from them may grow again. Either they have an additional radical introduced, or else they form a π bond, which can react with another radical and start growing again. When they do, it will again be a case of two chains coming together -- an active one and a macromonomer -- leading to a trmendous jump in molecular weight.
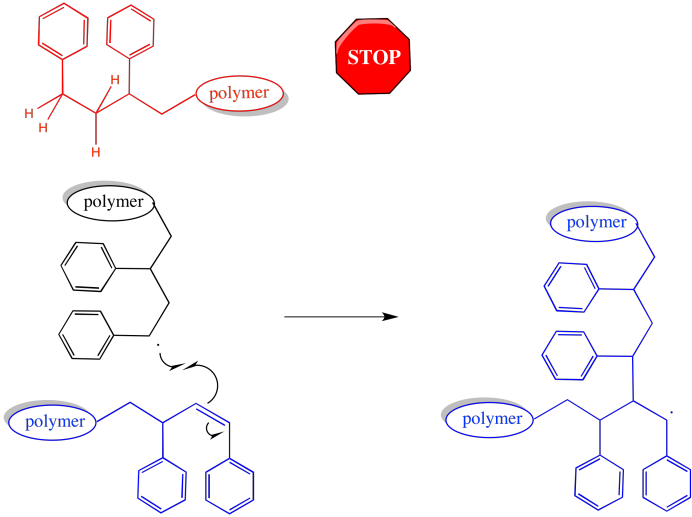
Figure RR9.7. Although one chain will stop growing after hydrogen atom abstraction, the other becomes a macromonomer, significantly broadening dispersity.
The morphology of the polymer is clearly altered by these events. Most growing chains are simply linear: they consist of a series of enchained monomers, all in one row. However, in either of the cases in which a growing chain has lost a hydrogen atom, then continued to grow, the shape will be branched.
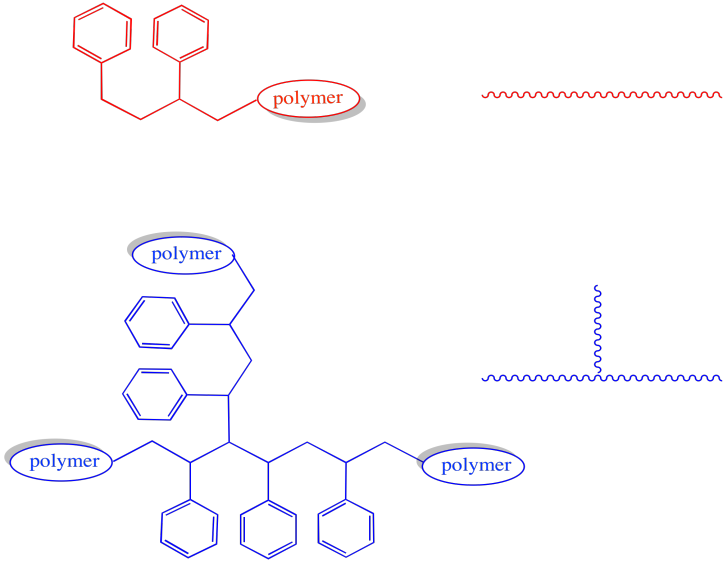
Figure RR9.8. A growing chain that has had a hydrogen abstracted from its backbone can start growing from two ends, leading to a branched structure.
These two architectures have very different properties. For example, we would expect a very different Tg in a branched polymer than a linear one, with chains flowing more freely at lower temperatures in the linear one.
Problem RR9.3.
It has been found that radical polymerization of styrene with a benzoyl peroxide initiator follows the rate law:
Rate = k [styrene][peroxide]1/2
Explain why this rate law occurs, based on the mechanism.
Problem RR9.4.
Provide mechanisms for radical formation from the following initiators:
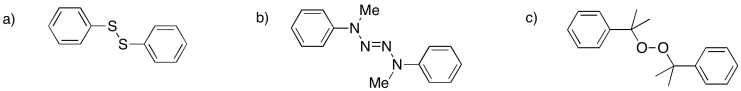
Problem RR9.5.
There is a great deal of interest in aqueous polymerizations for environmental reasons. Hydrogen peroxide is an obvious water-soluble initiator; however, it is slow to initiate polymerization. Show how initiation can be accelerated in the presence of Fe(II) ions.
Problem RR9.6.
A number of compounds can be used as inhibitors of radical polymerization. For example, benzoquinone will stop radical chains from growing. Show a mechanism for its reaction with a growing polymer chain and explain why the product of the reaction is a relatively stable radical.
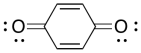
Problem RR9.7.
It may be surprising that one of the most effective inhibitors of radical polymerization is molecular oxygen; it can slow the polymerization rate by a factor of tens of thousands. The radical that results from the addition of O2 to a growing chain is considered to be resonance-stabilised. Show the mechanism of O2 addition and demonstrate resonance stability in the product.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: