Reactivity in Chemistry
Radical Reactions
RR2. Radical Initiation: Bond Homolysis
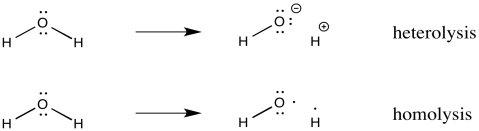
Sometimes, radicals form because a covalent bond simply splits in half. Two atoms that used to be bonded to each other go their separate ways. Each atom takes with it one electron from the former bond. This process is called homolysis, meaning the bond is breaking evenly. In contrast, heterolysis is the term for a bond that breaks via ionization, with one atom getting both electrons from the bond.

Figure RR2.1. The difference between bond heterolysis and bond homolysis.
Homolysis describes breaking a bond in half, with one electron going to each side of the former bond.
In pictures, we show this process using curved arrows, but the arrows we use are slightly different from the ones you may be used to seeing in polar reaction chemistry. Instead of a regular arrowhead, we use a half arrowhead. This kind of arrow looks a little more like a fish hook. It is easy to remember the roles of the two kinds of arrows, because a full arrowhead describes the movement of an electron pair, whereas a half arrowhead describes the movement of only one electron.
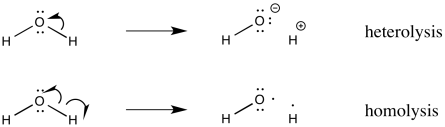
Figure RR2.2. Curved arrows illustrate electron movement in bond heterolysis and bond homolysis.
Why would a covalent bond simply break apart? There are really a number of factors and a number of events that may result in this situation. The simple part of the story is that the bond must have been weak in the first place. There was enough energy available in the form of heat transferred from the surroundings (or sometimes in the form of light) to overcome the stabilization energy of the bond.
What makes a bond weak or strong? That is a complicated question. Many factors influence bond strength. However, two of the main factors responsible for covalent bond strength are the degree of electron sharing because of "overlap" and the degree of bond polarity resulting from "exchange". Most strong covalent bonds rely on a mixture of these two factors.
One fairly common feature in homolysis is a bond between two atoms of the same kind. For example, elemental halogens often undergo homolysis pretty easily. The ease with which these bonds can be split in half is illustrated by their low bond dissociation energies. Not much energy needs to be added in order to overcome the bonds between these atoms.
| Bond | Bond Dissociation Energy (kcal/mol) |
| H-H | 105 |
| C-C | 85 |
| N-N | 65 |
| O-O | 47 |
| F-F | 37 |
| S-S | 45 |
| Cl-Cl | 57 |
| Br-Br | 45 |
| Sn-Sn | 45 |
| I-I | 35 |
This propensity for radical formation can be understood in terms of the lack of a polar component in these bonds. These atoms rely solely on atomic overlap to share electrons with each other.
There is a notable exception to the rule that homoatomic bonds are inherently weak, and that is a carbon-carbon bond. Its bond dissociation energy is listed in the table for comparison with the halogens. The relative strength of carbon-carbon bonds gives rise to a multitude of carbon-based "organic" compounds in nature. The formation of bonds between like atoms is called "catenation"; carbon is the world champion.
Of course, you can see from the table that hydrogen also forms a strong bond to itself, but since there is exactly one example of a resulting compound (H2) compared to the millions of examples of compounds containing C-C bonds, it's a relatively less common case.
Problem RR2.1.
Draw structures for the following reagents and show curved arrows to illustrate the initiation of radicals in each case.
a) Br2 b) H2O2 c) (CH3)3CO2H d) (CH3)2SbSb(CH3)2 e) CH3CH2CH2CH2SSCH2CH2CH2CH3
Silicon (BDESi-Si = 53 kcal/mol) and sulfur (BDES-S = 54 kcal/mol) are also capable of catenation, but the bonds that these atoms form between themselves are much weaker than C-C bonds. It seems to be generally true that larger atoms form weaker bonds, at least in the main group of the periodic table. After all, I-I bonds are weaker than Br-Br bonds, which are weaker than Cl-Cl bonds.
It is sometimes argued that this trend is a result of poor spatial overlap between the more diffuse p orbitals nearer the bottom of the periodic table. However, quantum mechanical calculations indicate that isn't necessarily the case. On the other hand, the other side of the equation must not be ignored. Once these bonds break, two new radicals form. Just as ions are more stable on larger, more polarizable atoms, so are radicals.
Radicals are more stable on larger, more polarizable atoms.
For example, sulfur radicals are more stable than oxygen radicals.
These trends show up in a comparison of carbon-halogen bond strengths. The average carbon-iodine bond is much weaker than the average carbon-fluorine bond.
| Bond | BDE (kcal/mol) |
| C-F | 116 |
| C-Cl | 78 |
| C-Br | 68 |
| C-I | 51 |
Other factors that stabilize radicals can also tilt events in favor of bond homolysis. For example, during catalytic hydrogenations, ether linkages at benzylic positions are often cleaved. A C-O bond is not inherently weak, but a benzylic radical is quite stable. It is the stability of the resulting radical that weakens this particular C-O bond and allows it to be broken so easily.
Particularly stable radicals form relatively easily.
For example, benzylic radicals form very easily.
Some compounds are commonly used as radical initiators. For example, peroxides contain weak O-O bonds that can cleave to form radicals. That's the initial event in the formation of a radical from benzoyl peroxide, but the resulting carboxyl radical quickly decomposes in favour for carbon dioxide formation.
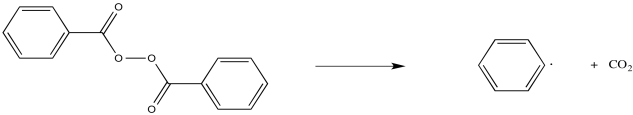
Figure RR2.3. Radical initiation by benzoyl peroxide.
AIBN, on the other hand, can cleave to produce a very strong dinitrogen triple bond, leaving behind two radicals.
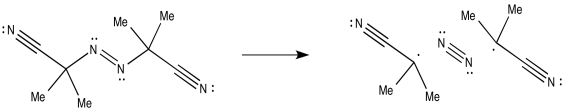
Figure RR2.4. Radical initiation by azoisobutyronitrile (AIBN).
DMPA is a photoinitiator; it is cleaved by the addition of light.
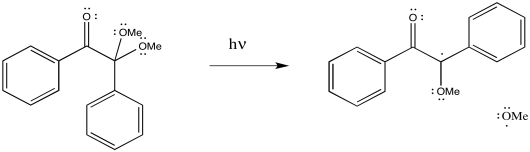
Figure RR2.5. Radical initiation by dimethoxyphenylacetophenone (DMPA).
Problem RR2.2.
Provide mechanisms for radical formation from
a) benzoyl peroxide b) AIBN c) DMPA
Problem RR2.3.
The following initiators form radicals relatively easily. Provide mechanisms for radical formation in each case.
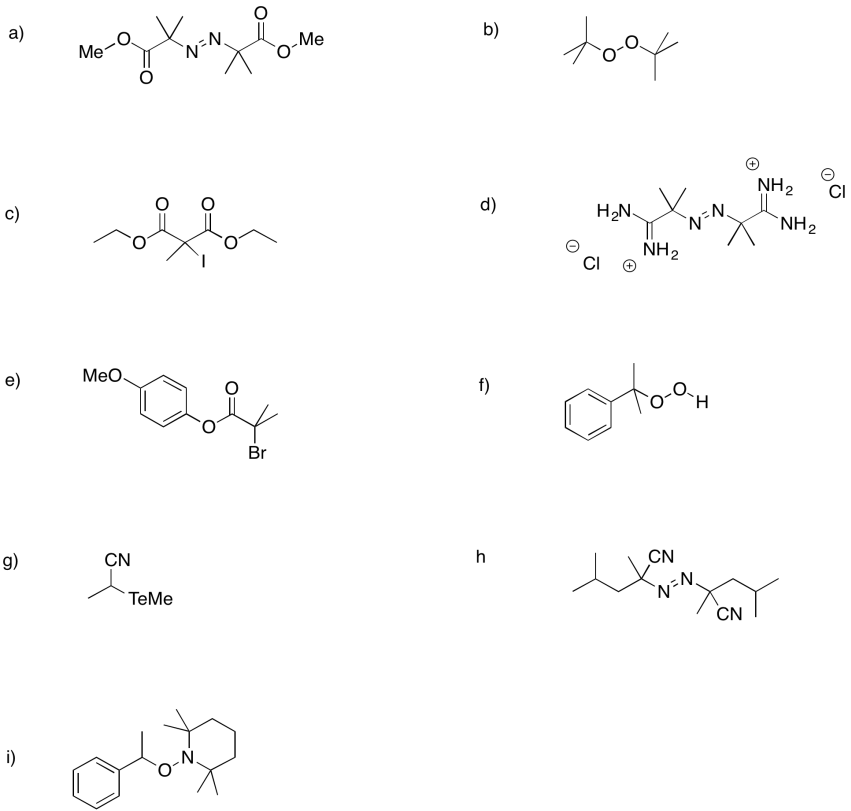
Problem RR2.4.
Chemists have a wide range of initiators available. The following two examples are similar in some ways, but may be useful under different conditions. For what conditions might each initiator best be suited?

More information on bond strengths is available at Wired Chemist.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: