Reactivity in Chemistry
Radical Reactions
RR1. Introduction to Radicals
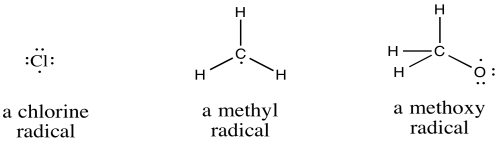
Radicals are species that have unpaired electrons. They can be atoms or molecules and they can be neutral species or ions. Frequently, radicals are very reactive. However, their mode of reactivity does not fall neatly within the normal patterns of Lewis acids and bases, or nucleophiles and electrophiles.

Figure RR1.1. Examples of radicals.
Radicals play common roles in atmospheric chemistry, including equilibration of the ozone layer. They are also found in a variety of biochemical pathways. In addition, radicals are employed in a number of useful processes, such as the polymerization of methyl methacrylate or vinyl chloride, commonly used to make shatter-resistant "glass" and pipes for plumbing, respectively.
Compounds of p-block elements form radicals if one of the atoms has seven electrons in its valence shell rather than the usual eight.
Problem RR1.1.
Draw structures for the following neutral radicals, making sure to fill in the correct number of electrons.
a) Br b) OH c) CH3CH2 d) CH3CH2S e) CH2CHCH2 f) NO g) (CH3)2NO h) NO2
A molecule could become a radical in a number of different ways. A bond may break in half via the addition of energy, in the form of either heat or light. Otherwise, it may simply transfer one of its electrons elsewhere. Again, this event may be precipitated by the addition of heat or light energy. Of course, a molecule that receives an additional electron from elsewhere may also become a radical.
Problem RR1.2.
Draw structures for the following cationic radicals, making sure to fill in the correct number of electrons and the formal charge.
a) H2C=O b) CH3NH2 c) CH3OCH3 d) CH3CH2CH2Br e) CH3CH2CHCH2
Problem RR1.3.
Draw structures for the following anionic radicals, making sure to fill in the correct number of electrons.
a) O2 b) H2CO c) CH3CCCH3 d) cyclo-C6H6
The compounds above are all simple radicals, containing one unpaired electron. Compounds may also have more than one unpaired electron.
Elemental oxygen, O2, is a diradical. Although its Lewis structure does not suggest anything unusual, its molecular orbital diagram reveals that oxygen actually has two unpaired electrons.
Problem RR1.4.
Show two Lewis structures for O2: one illustrating its double bond, and the other illustrating its diradical character.
Problem RR1.5.
Show, with a molecular orbital interaction diagram, the diradical character of dioxygen.
A diradical could take two different forms. For example, molecular oxygen has two singly-occupied molecular orbitals. The single electron in each of those orbitals could adopt one of two different spin states. Both could adopt the same spin state (designated with arrow "up", for example), or they could be "spin-paired" (one "up", one "down"). The former situation is called a "triplet state", whereas the latter case is termed a "singlet state". These two situations result in some physical differences, such as different interactions with a magnetic field.
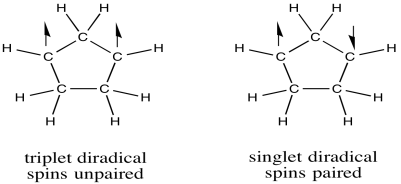
Figure RR1.2. Triplet (left) and singlet (right) diradicals.
Subsequent pages will focus on the reactivity of radicals, with an emphasis on the stages of radical chain reactions.
Problem RR1.6.
Some transition metal compounds have radical characteristics. Show why, using a low spin Co(II) complex in an octahedral environment as an example.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: