Reactivity in Chemistry
Radical Reactions
RR7. Radical Substitution
There are a couple of classic reactions based on radical chemistry that are often used to illustrate different consequences of the mechanism. One of these reactions is the radical substitution of a halogen atom for a hydrogen atom.

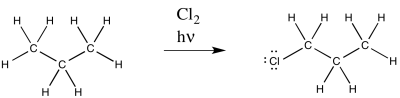
Figure RR7.1. A radical chlorination of an alkane.
This reaction is commonly observed with chlorine and bromine. Multiple products are possible; only one of these products is shown above.
As a radical reaction, the first step would have to be an initiation step. If the reaction proceeds as written, via the addition of molecular chlorine and light, then initiation would involve direct homolysis of the chlorine-chlorine bond. The light provides enough energy to break the relatively weak chlorine-chlorine bond.
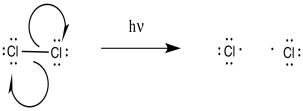
Figure RR7.2. Initiation of chlorine radicals.
Once the chlorine radicals are present, propagation steps would occur. Given the ultimate replacement of a hydrogen by a chlorine atom, radical abstraction of a hydrogen atom seems likely. That event would lead to the formation of an alkyl radical.
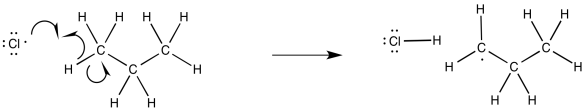
Figure RR7.3. Propagation via hydrogen atom abstraction from an alkane.
The newly-formed alkyl radical would be able to engage in propagation reactions as well. For example, it could react with another chlorine molecule. It would produce a new chlorine radical and the reaction product, chloroproane.
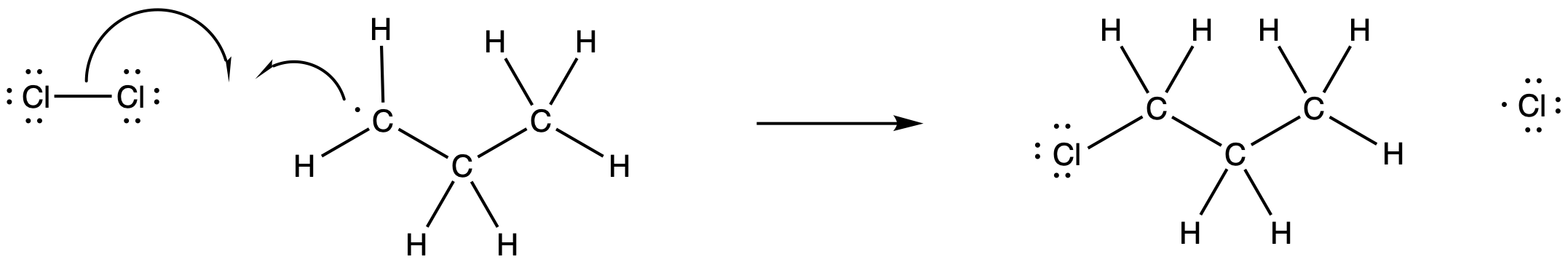
Figure RR7.4. Propagation via halogen atom abstraction.
Alternatively, if we wanted to imagine the full range of elementary steps in radical reactions, we could picture a termination step, with another chlorine combining with that alkyl radical to form a chloroalkane product.
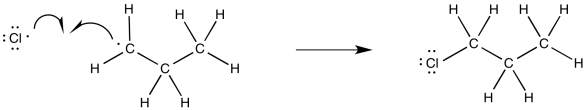
Figure RR7.5. A possible termination step during radical chlorination.
Problem RR7.1.
Propose a mechanism, with curved arrows, for the formation of 2-chloropropane from propane in the presence of chlorine radicals.
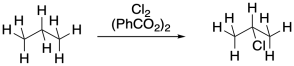
Problem RR7.2.
Propose a mechanism, with curved arrows, for the following variation on radical chlorination.
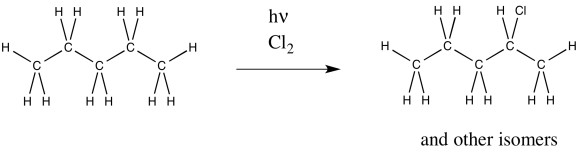
Problem RR7.3.
Radical chlorination of pentane also results in multiple products. One of them is shown below. What other isomers are formed?
Clearly, radical halogenation could result in a mixture of products. That's because there are different hydrogen atoms that could be extracted in the first propagation step. Abstracting a hydrogen atom from the middle carbon of propane would lead ultimately to 2-chloropropane. Abstracting a hydrogen atom from either of the end carbons of propane would lead to formation of 1-chloropropane. Consequently, radical halogenations of this sort often result in mixtures of products.
In reactions of carbocations, we can usually predict which products will result because we know something about carbocation stability. We know that cations at more-substituted positions are more stable than cations at less-substituted positions, and so in reactions that proceed through carbocations we frequently see a nucleophile incorporated at the more-substituted position. Radicals display a similar trend in stability, but it is much more subtle. We don't see the new atom exclusively at the more-substituted position, but we may see more of it there than in other places, because the radical intermediate is slightly more stable there than at less-substituted positions.
The effect of radical stability is subtle enough that other factors can easily influence ratios of products in reaction mixtures. Temperature becomes very important. At lower temperatures, there may be just enough energy to form the more stable radical, whereas other positions are just as likely to form radicals when things warm up.
Different reagents can also give different product profiles. Chlorine radicals, for example, are more reactive than bromine radicals. That added reactivity leaves them more likely to react randomly. Bromine radicals, in contrast, are a little more stable and so they react a little more selectively. A radical substitution using bromine results in more product forming at the more-substituted position compared to a radical substitution using chlorine.
Problem RR7.4.
Which product would be the major one in radical bromination of propane? Why?
In other cases, radical substitutions proceed with a much higher degree of selectivity. In compounds containing double bonds, a C-H next to the double bond, in what is called the allylic position, is particularly susceptible to radical hydrogen abstraction. That's because of the resonance stability of the allylic radical. The stability of this radical makes it form more easily.
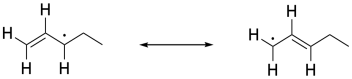
Figure RR7.6. An example of an allyl radical.
In a radical substitution reaction, replacement of a hydrogen atom at an allylic position is much faster than other positions in the molecules. Resonance delocalization is really the dominant factor in radical stability, so the possibility of a radical in an allylic position presents a much lower-energy pathway for the reaction.
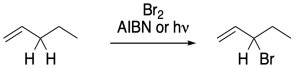
Figure RR7.7. Allylic bromination.
Allylic halogenations can be initiated by light, as we saw above, or we can use other radical initiators, such as AIBN or benzoyl peroxide. The radical reaction is highly selective at the allylic position. Unfortunately, there is a competing polar reaction that occurs at the double bond in the presence of bromine.
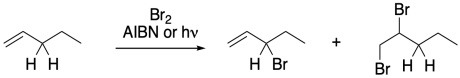
Figure RR7.8. Competing bromination reactions.
To suppress that competing reaction, the bromine concentration is often kept to a minimun through the use of an alternative reagent, such as N-bromosuccinimide (NBS). NBS is a source of bromine in solution. It works well in conjunction with radical initiators such as AIBN.
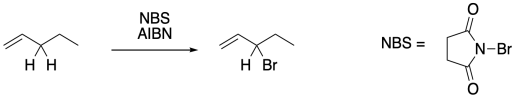
Figure RR7.9. Allylic bromination using NBS.
Radical reactions also occur very well at benzylic positions. Benzylic hydrogens are those attached to a carbon next to a benzene ring. Like allylic hydrogens, benzylic hydrogens are easily abstracted because of the stability of the radical that results.
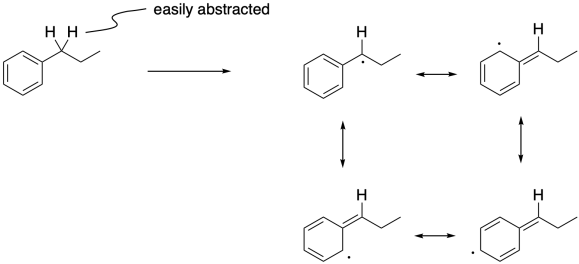
Figure RR7.10. Benzylic radical can be formed via hydrogen atom abstraction.
Benzylic bromination is very similar to allylic bromination because of that radical stability. It can be done in exactly the same way, with some NBS and a radical initiator.
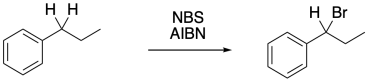
Figure RR7.11. Benzylic bromination.
The stability of the benzylic radical also facilitates other reactions. Perhaps the most notable is benzylic oxidation, which occurs specifically at the carbon next to a benzene ring and requires a C-H bond at that position.
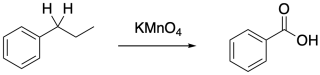
Figure RR7.7. Benzylic oxidation.
Problem RR7.5.
Fill in the reagents for the following reactions.
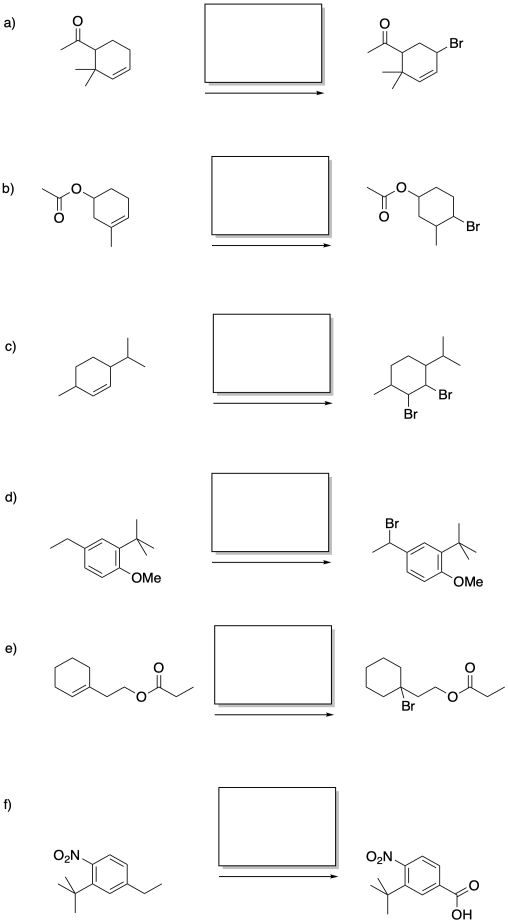
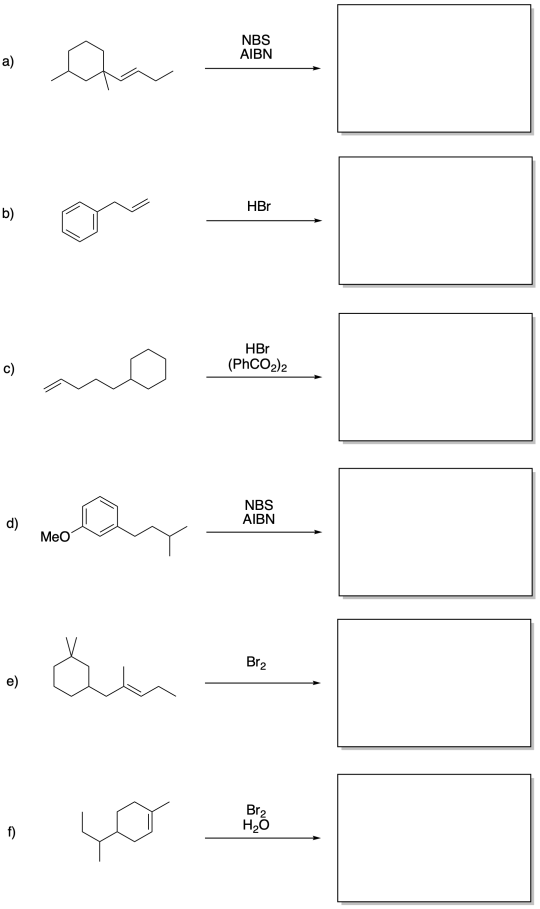
Problem RR7.6.
Fill in the products of the following reactions.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: