Reactivity in Chemistry
Radical Reactions
RR6. Radical Termination
If propagation steps lead to chain reactions, continually leading to the formation of new radicals that engage in further propagation reactions, then where does it end? That self-perpetuating cycle can be part of the beauty of radical reactions, allowing a very small amount of initiator to efficiently start a process that then sustains itself. However, it is important that the reaction has an end point, so that it does not continue to run out of control.
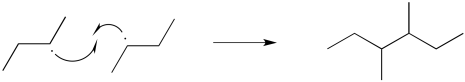
There are a couple of different ways that radical reactions commonly come to an end. These elementary reactions are called terminations steps. The simplest event that could occur is a radical recombination step. In that case, two radicals approach each other and bond together, sharing their previously unpaired electrons.

Figure RR6.1. Radical coupling: a termination step in a radical chain reaction.
In termination steps, two radicals come together to make no radicals.
Energetically, termination steps like this one should be pretty favourable, because they involve formation of a new bond. These steps are mostly limited by the low concentration of radicals in solution. If two radicals don't happen to bump into each other, then they won't react together.
A second event leading to termination also involves the collision of two radicals. However, the trajectory of the two radicals is slightly different, so that the unpaired electrons do not connect with each other. Instead, the unpaired electron on one radical molecule encounters a hydrogen on the other molecule. If the hydrogen is alpha to the radical, it is easily abstracted, forming a double bond.
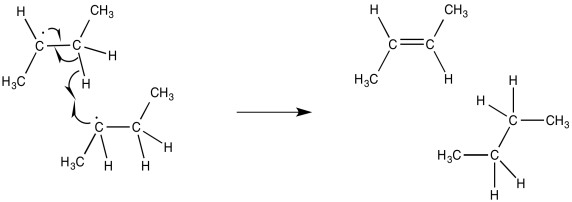
Figure RR6.2. Radical elimination: termination via hydrogen atom abstraction in a radical chain reaction.
Researchers are actually able to measure the bond strength of such a hydrogen alpha to a radical. They find that the bond is considerably weakened compared to a similar hydrogen atom in a molecule that does not contain a radical in the same position.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: