Reactivity in Chemistry
Electrophilic Rearrangement
ER4. Beckmann Rearrangement
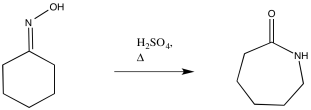
The Beckmann rearrangement results when an oxime (an N-hydroxyimine, C=N-OH) is treated with concentrated acid and heated. The product of the reaction is an amide (C-N=O). In this case, a 6-membered ring turns into a 7-membered ring when the carbon attached to the imine carbon shifts over to bond to the imine nitrogen. The OH group becomes the oxygen of the carbonyl.

Figure ER4.1. A Beckman rearrangement transforms an oxime into an amide.
The oxime, in turn, is generated by treatment of a ketone with hydroxylamine. A catalytic amount of acid can activate the carbonyl, accelerating the otherwise sluggish reaction.
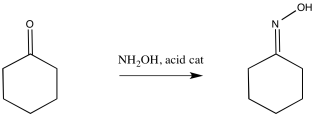
Figure ER4.2. Formation of an oxime from a ketone.
The Beckmann rearrangement, like the pinacol rearrangement, is triggered by the loss of water from the starting material. The incipient cation that results undergoes a 1,2-shift. We say "incipient" cation meaning the cation never actually forms. Instead, the partial positive charge that develops on nitrogen when the attached oxygen is protonated is enough to draw the neighbouring carbon over in a 1,2-shift. That donation to the nitrogen displaces or pushes out the water that was attached to the nitrogen. Subsequently, the re-addition of water to the rearranged cation results in a new compound. Nevertheless, if a cation did form on nitrogen, the reaction would look like the drawing below.
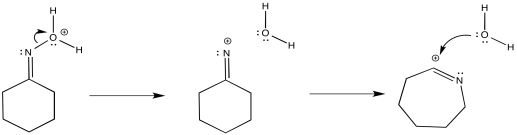
Figure ER4.3. An illustration of the electrostatics behind the Beckmann rearrangement.
Once again, the drawing above is just meant to reinforce the link between this rearrangement and the more familiar 1,2-hydride chift. By looking at cation formation, we can see why a bond might shift to replace a less stable nitrogen cation with a relatively more stable carbocation. It's not quite the real picture. Remember, by "incipient", we describe a cation that is only on the brink of forming, but has not actually occurred yet. The 1,2-shift happens as soon as the partial positive charge on the nitrogen becomes great enough to draw the electrons from the neighbouring bond. The nitrogen becomes positive because it is attached to a more electronegative oxygen atom, and the oxygen just got protonated, taking on a positive charge. The next drawing depicts the more subtle reality of this step in the mechanism.
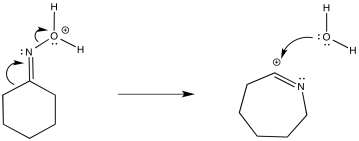
Figure EA4.4.The concerted 1,2-shift and water displacement in a Beckmann rearrangement.
The complete mechanism is described pictorially below. Here, the proton source is shown as H2SO4 and the base in the system is shown as bisulfate anion, HSO4-, but really the base is anything with a lone pair, and likely it's another water molecule. Note the enol-like donation of the oxygen lone pair to the π-bond to form the carbonyl.
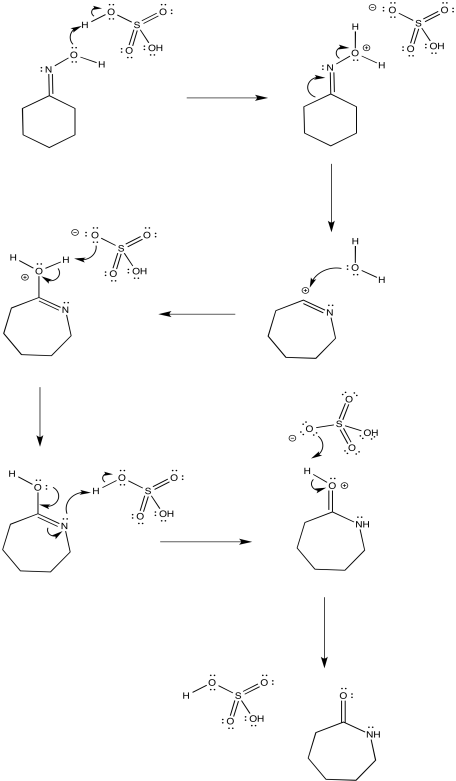
Figure EA4.5.The complete mechanism of a Beckmann rearrangement.
A Beckmann rearrangement requires pretty srongly acidic conditions, which can be provided by sulfuric acid. The reaction can also be carried out with strong Lewis acids. A strong Lewis acid, such as AlCl3, is capable of polarizing the oxime enough to induce the rearrangement.
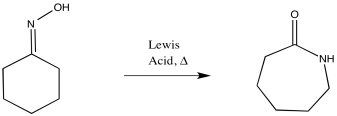
Figure EA4.6.A Lewis acid-induced Beckmann rearrangement.
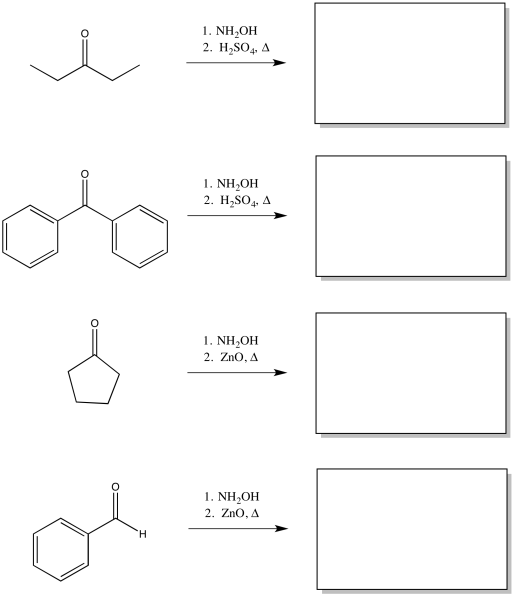
Problem ER4.1.
Predict the products of the following Beckmann rearrangements.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: