Reactivity in Chemistry
Electrophilic Rearrangement
ER2. Pinacol Rearrangement
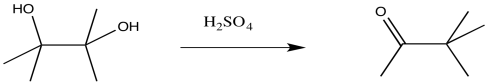
The pinacol rearrangement is a reaction of 1,2-diols. It takes place under the influence of strong acids, inluding mineral acids like sulfuric acid. It can also be brought about via the use of Lewis acids. The reaction overall involves the loss of one of the hydroxyl groups, the conversion of the other hydroxyl group into a carbonyl, and the shift of an alkyl group.

Figure ER2.1 A pinacol rearrangement.
The heart of the rearraction is the 1,2-shift of an alkyl group. This shift is assisted by π-donation from the oxygen, converting the C-O bond into a carbonyl at the same time.
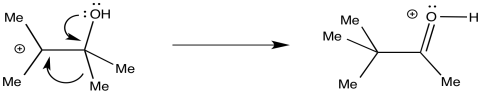
Figure ER2.2. The central 1,2-shift step of a pinacol rearrangement.
The entire mechanism would look like something like the one below. In this illustration, the diol is protonated by H2SO4 directly, producing a bisulfate conjugate base, HSO4-. Sulfuric acid is always aqueous (because it is produced by the reaction of water with sulfur trioxide), so this step is often shown as protonation by H3O+, forming water as the conjugate base. Then, water would be the base that deprotonates the OH group to make a ketone, rather than deprotonation by bisulfate ion.
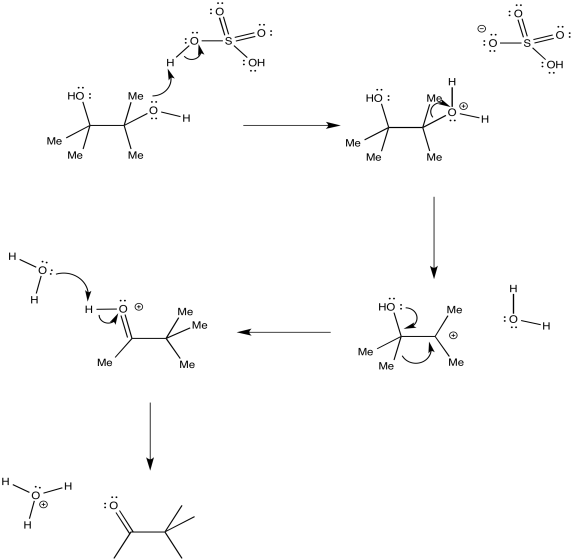
Figure ER2.3. More mechanistic details of the pinacol rearrangement.
In many rearrangements, formation of the actual cation is not necessarily involved. The 1,2-shift could occur as the water molecule leaves. The protonated oxygen may draw enough electron density toward itself to induce the shift.
Frequently there is a fine line between alternative reaction pathways. In many cases, the stability of an intermediate determines whether a reaction happens in stages or in one step. If the intermediate is relatively stable, the reaction will happen in steps. The less stable the intermediate, the more likely the reaction will proceed in a concerted manner. For example, SN2 reactions proceed in one step. In most cases, that's because the carbocation intermediate is too unstable to form.
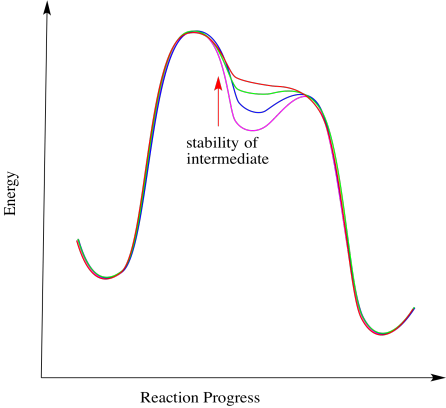
Figure ER2.4 The relationship between intermediate stability and the presence of intermediates.
In the pinacol reaction shown, a tertiary carbocation is forming, which should be relatively stable. However, the reaction could still proceed without that intermediate for a different reason. If the barrier for the subsequent step is very low, then the lifetime of the intermediate will get shorter. The more that barrier drops, the less time the intermediate lasts, until it really doesn't form at all. At that point, this is a one-step reaction rather than a two-step reaction.
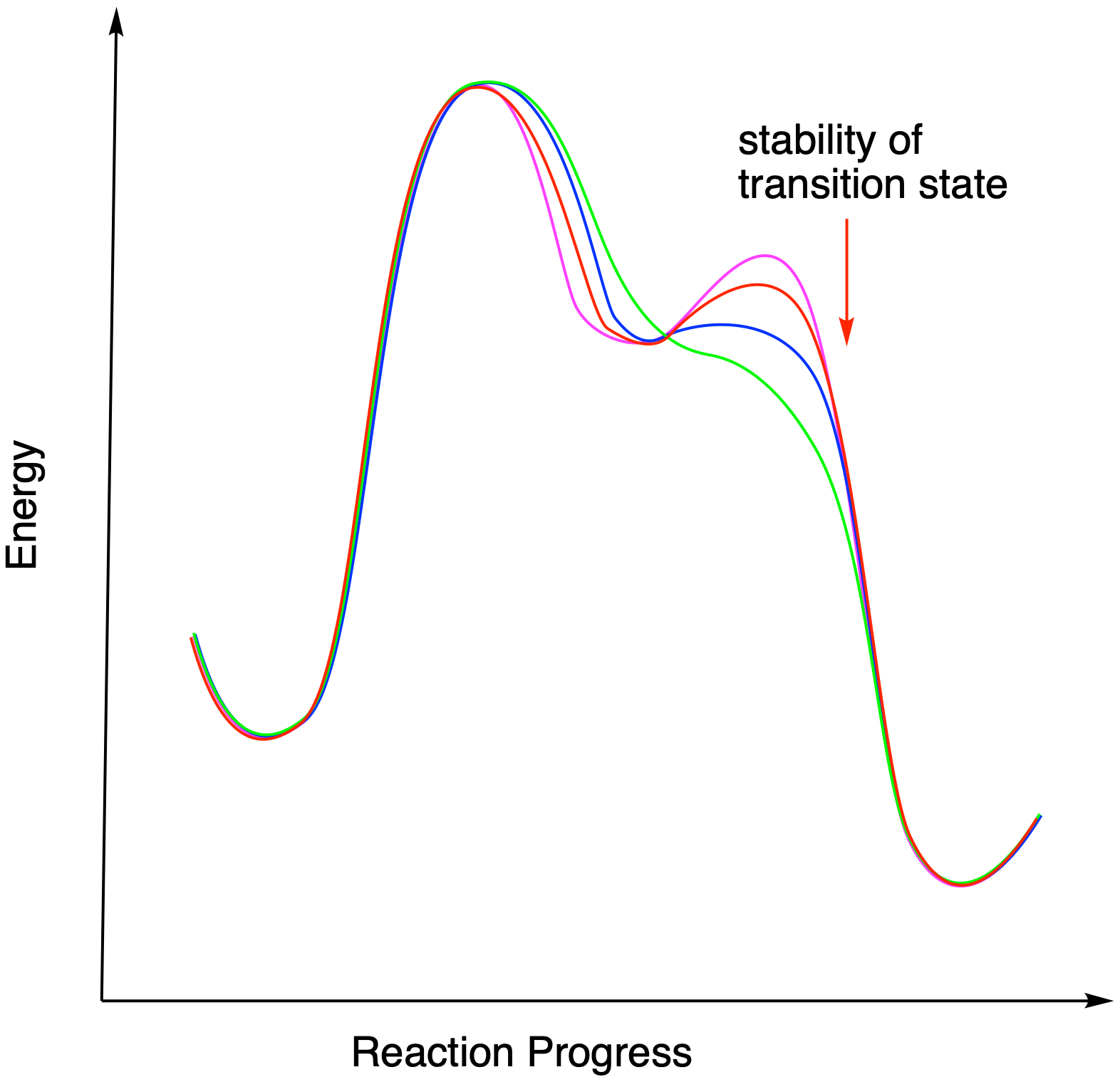
Figure ER2.5. The relationship between the barrier to a second step and the presence or absence of an intermediate.
The barrier to the 1,2-shift in the pinacol reaction may be lowered, and that step accelerated, by the π-donation from the oxygen. We have often seen that π-donation is both an important stabilizing factor in intermediates and it is also an important factor in lowering barriers and activation molecules toward certain reaction steps.
Problem ER2.1.
Fill in the products of the following pinacol rearrangements.
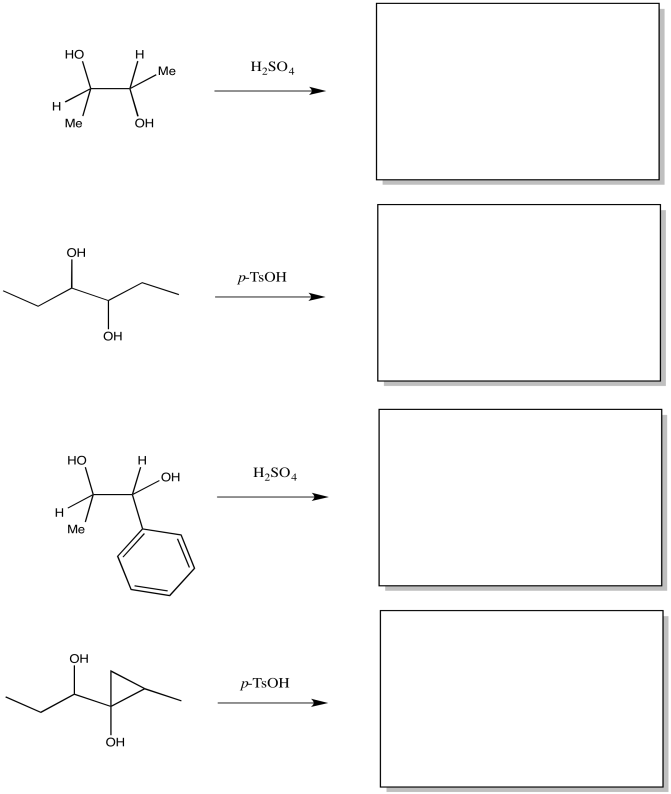
Problem ER1.2.
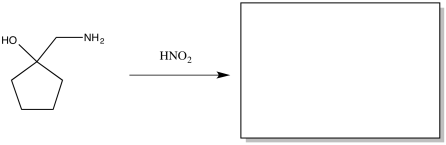
The Tiffeneau Demjanov rearrangement is superficially similar to a pinacol rearrangement. It occurs when a β-amino alcohol is treated with nitrous acid. The reaction results in loss of the amino group with rearrangement of the molecule. Predict the product of the reaction.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: