Reactivity in Chemistry
Electrophilic Rearrangement
ER5. Wolff Rearrangement
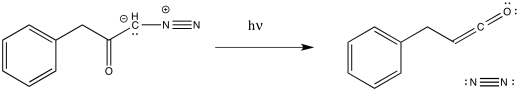
The Wolff rearrangement is the conversion of a diazoketone to a ketene, usually under photolytic conditions. The reaction can also be brought about by heating or through the addition of Ag(I) salts.

Figure ER5.1 The Wolff rearrangement.
The loss of dinitrogen from the diazonium compound would result in an electron-deficient carbene. Like a carbocation, the carbene would be susceptible to a 1,2-shift. If accompanied by π-donation from the carbene to the carbonyl, a ketene would result.
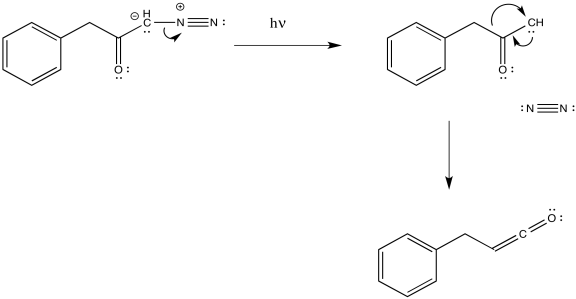
Figure ER5.2. A possible mechanism for the Wolff rearrangement.
As with other rearrangements, the 1,2-shift could occur at the same time as the loss of the dinitrogen. That could happen because of the relative instability of the carbene. It could also result from the π-donation by the carbanion, which is a strong donor. As we have seen before, π- donation can accelerate 1,2-shifts.
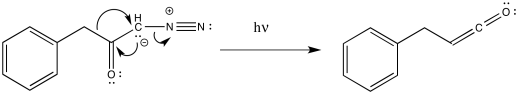
Figure ER5.3. The Wolff rearrangement probably proceeds with concerted dinitrogen loss and 1,2-shift.
So, the product of that reaction is a ketene, containing a C=C=O unit. Ketenes are not terribly stable. In the presence of nucleophilic solvents such as water or alcohol, the ketene easily undergoes nucleophilic addition. Addition of water would result in a carboxylic acid. Addition of an alcohol would result in formation of an ester.
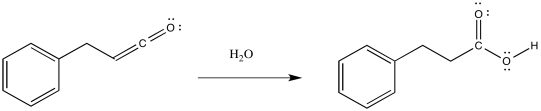
Figure ER5.4. Formation of a carboxylic acid via Wolff addition in water.
The mechanism of that addition involves keto-enol tautomerism. Remember, an OH group attached to a carbon-carbon double bond has a little enhanced acidity because of conjugation effects.
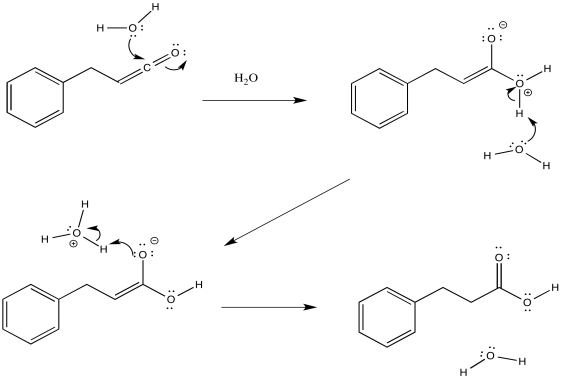
Figure ER5.5. The addition of water to ketene forms a carboxylic acid.
The Arndt-Eistert reaction is a useful application of the Wolff rearrangement. This reaction is used to take a carboxylic acid and make its chain one carbon longer by inserting a CH2 group into the chain. It is a three-step process, involving the sequential addition of thionyl chloride, then diazomethane, and finally silver(I) nitrate.
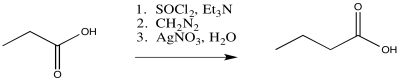
Figure ER5.6. The Arndt-Eistert reaction.
That first step is used to convert the carboxylic acid into an acid chloride. Thionyl chloride, SOCl2, is often used for these purposes. The same step can also be accomplished using oxalyl chloride, (COCl)2, which is a somewhat gentler reagent than thionyl chloride.
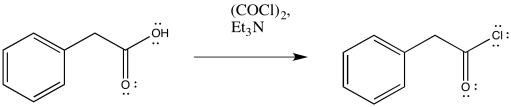
Figure ER5.7. Conversion of a carboxylic acid to an acid chloride using oxalyl chloride.
The acid chloride is then converted to a diazoketone through the addition of diazomethane, CH2N2. This is not a trivial step because diazomethane, and diazo compounds in general, can be highly explosive.
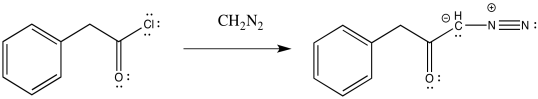
Figure ER5.8. Conversion of an acid chloride into a diazoketone using diazomethane.
The conversion of an acid chloride into a diazoketone is sometimes carried out in the presence of a mild base. The base absorbs or neutralizes the HCl produced as a by-product of the reaction. For example, one set of conditions for this reaction uses calcium oxide or "quicklime", one of the ingredients in cement.
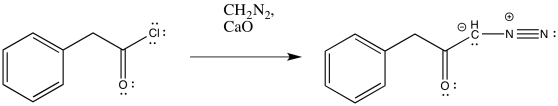
Figure ER5.9. Conversion of an acid chloride into a diazoketone in the presence of base.
Finally, we get to the third step in the Arndt-Eistert reaction, which is the Wolff rearrangement itself. As noted earlier, this step could be carried out photolytically. In principle, it could also be carried out with heating, as shown here.
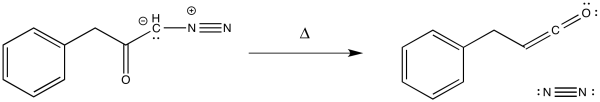
Figure EA5.10. Wolff rearrangement induced by heat.
Heating a reaction to which you have recently added diazomethane may be a little frightening because of the exploside properties mentioned earlier. It's useful to have milder, more elegant methods of carrying reactions out rather than hitting them with a hammer (or torching them with a Bunsen burner, if anyone still uses Bunsen burners in organic laboratories). Silver salts are often used to gently trigger decomposition of the diazoketone.
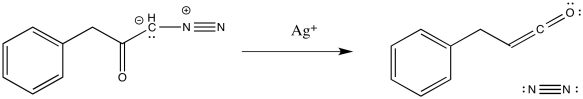
Figure EA5.11. Wolff rearrangement promoted by the addition of silver(I).
Of course, if these silver salts are dissolved in water, then the ketene is immedately captured by water. An enol-keton tautomerization quickly follows, leading to formation of a carboxylic acid.
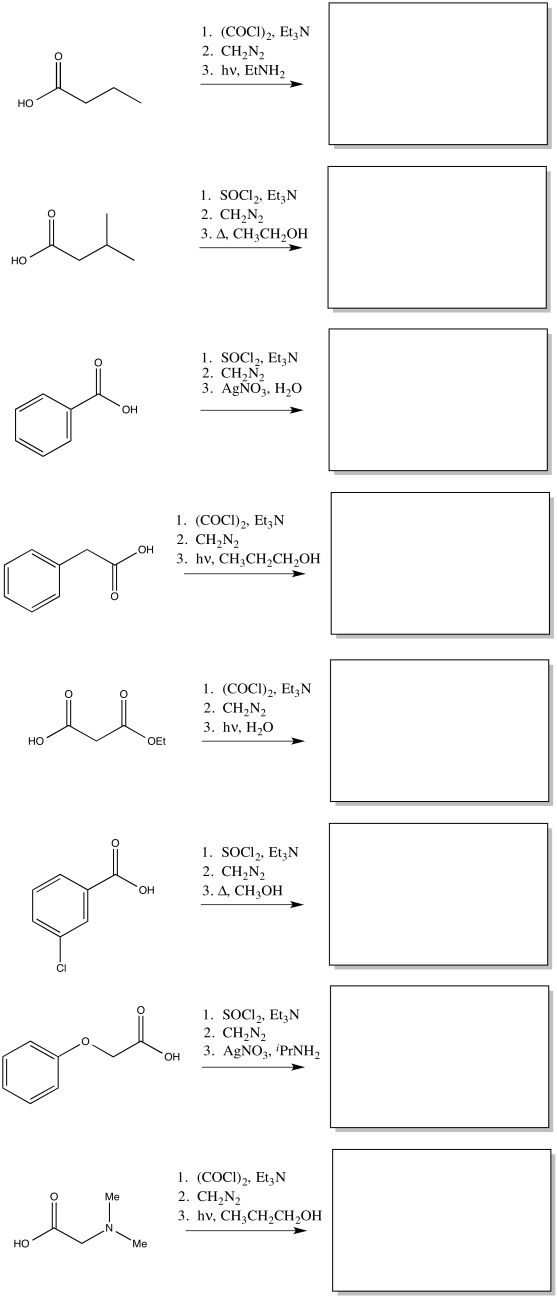
Problem ER5.1.
Predict the products of the following Wolff rearrangements.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation: