AB1. General Acidity & Basicity
Physical changes result in the transition of a molecular material from one form into another without any change in the composition of the material. A liquid compound can be cooled until it freezes or heated until it evaporates, but the atoms that make up the material are still connected together in the same way.
Chemical reactions result in a change in the composition of a material. Atoms become associated in different ways. Changes in bonding occur. Because bonding involves some kind of shared distribution of electrons between atoms, a chemical reaction involves some change in how electrons are arranged in the material.
- The standard way to think about a chemical reaction is to consider the movement of electrons in the reaction.
Reactions often involve many changes, so that one material is transformed into others via numerous redistributions of electrons. These individual steps within an overall reaction are sometimes called elementary reactions. One of the most common ways to analyze an elementary reaction is to understand in a very basic way where the electrons are moving from and where they are moving towards.

Figure AB1.1. Electron movement and bond formation in a generalized reaction. Here, atom B is donating a pair of electrons to atom A.
- An atom or molecule that supplies a pair of electrons to form a new bond is an electron donor. An electron donor is often called a Lewis base.
- An atom or molecule that accepts a pair of electrons to form a new bond is an electron acceptor. An electron acceptor is often called a Lewis acid.
One of the most common Lewis acids, or electron acceptors, is a proton. It is so common that people often use the term "acid" just to describe compounds or solutions that supply protons.

Figure AB1.2. Proton as Lewis acid.
A Lewis base is anything with a lone pair. For example, water is a Lewis base because the oxygen atom has lone pairs. If we take a source of protons, such as hydrogen chloride (HCl, a colourless gas) and bubble it into a flask of water, a reaction will result. The water will donate electrons to the proton in the HCl, producing hydrochloric acid, or aqueous hydrogen chloride: HCl(aq).

Figure AB1.3. Donation of electrons from a water molecule to a proton./p>
Note that a hydrogen atom can only have one bond, so when the water donates its electrons to the hydrogen, the bond between the hydrogen and the chlorine has to be broken. The electrons in the bond move to the chlorine, which is more electronegative than the hydrogen.
Also, notice that the hydrogen atom did not even need a full positive charge to attract the water. A partial positive charge will still attract electrons.
Acids don't have to be protons or protons donors. Very often, metal ions are able to attract electrons, because metal ions are positively charged. Ca2+, Sc2+, Ti4+ are just a few common examples.
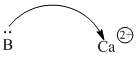
Figure AB1.4. A calcium ion can attract electrons from a donor atom.
These ideas will be explored more fully in this chapter. They are some of the most important ideas in chemistry, forming the basis for most of what we know about the relationships between structure, properties, and reactivity.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: