AB9. Proton Donor Strength: pKa
It is helpful to have a way of comparing Bronsted-Lowry acidities of different compounds. If the chemistry of protons involves being passed from a more acidic site to a less acidic site, then the site that binds the proton more tightly will retain the proton, and the site that binds protons less tightly will lose the proton. If we know which sites bind protons more tightly, we can predict in which direction a proton will be transferred.
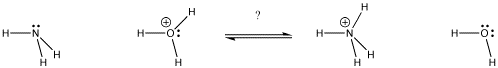
Figure AB9.1. In which direction will the equilibrium lie? Which base gets the proton?
There is an experimentally-determined parameter that tells us how tightly protons are bound to different compounds. "Experimental" often implies to students "untested" or "unreliable", but here it means that someone has done the work to measure how tightly the proton is bound. Experimental in this sense means "based on physical evidence".
This experimental parameter is called "the pKa". The pKa measures how tightly a proton is held by a Bronsted acid. A pKa may be a small, negative number, such as -3 or -5. It may be a larger, positive number, such as 30 or 50. The lower the pKa of a Bronsted acid, the more easily it gives up its proton. The higher the pKa of a Bronsted acid, the more tightly the proton is held, and the less easily the proton is given up.
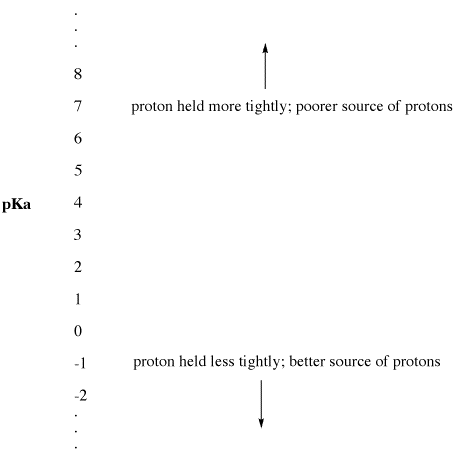
Figure AB9.2. The pKa scale as an index of proton availability.
- Low pKa means a proton is not held tightly.
- pKa can sometimes be so low that it is a negative number!
- High pKa means a proton is held tightly.
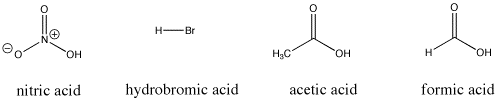
Figure AB9.3.> Some Bronsted acidic compounds; these compounds all supply protons relatively easily.
For example, nitric acid and hydrochloric acid both give up their protons very easily. Nitric acid in water has a pKa of -1.3 and hydrobromic acid has a pKa of -9.0. On the other hand, acetic acid (found in vinegar) and formic acid (the irritant in ant and bee stings) will also give up protons, but hold them a little more tightly. Their pKas are reported as 4.76 and 3.77, respectively. Water can certainly give up a proton, but not very easily; it has a pKa of around 14. Methane is not really an acid at all, and it has an estimated pKa of about 50.

Figure AB9.4. Some not-so-acidic compounds. Water is very, very weakly acidic; methane is not really acidic at all.
The pKa measures the "strength" of a Bronsted acid. A proton, H+, is a strong Lewis acid; it attracts electron pairs very effectively, so much so that it is almost always attached to an electron donor. A strong Bronsted acid is a compound that gives up its proton very easily.
A weak Bronsted acid is one that gives up its proton with more difficulty. Going to a farther extreme, a compound from which it is very, very difficult to remove a proton is not considered to be an acid at all.
When a compound gives up a proton, it retains the electron pair that it formerly shared with the proton. It becomes a conjugate base. Looked at another way, a strong Bronsted acid gives up a proton easily, becoming a weak Bronsted base. The Bronsted base does not easily form a bond to the proton. It is not good at donating its electron pair to a proton. It does so only weakly.
In a similar way, if a compound gives up a proton and becomes a strong base, the base will readily take the proton back again. Effectively, the strong base competes so well for the proton that the compound remains protonated. The compound remains a Bronsted acid rather than ionizing and becoming the strong conjugate base. It is a weak Bronsted acid.
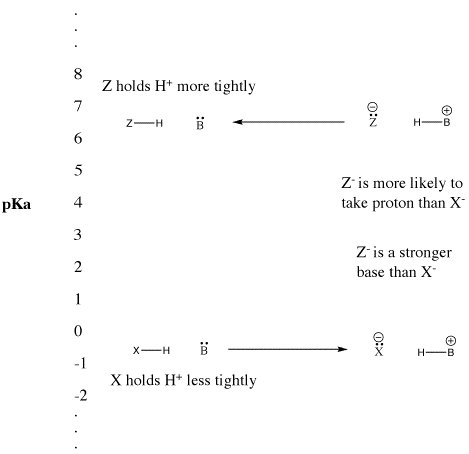
Figure AB9.5. The pKa scale and its effect on conjugate bases.
- "Strong" Bronsted acids ionize easily to provide H+.
- This term is usually used to describe common acids such as sulfuric acid and hydrobromic acid.
- "Weak" Bronsted acids do not ionize as easily.
- This term is often used to describe common acids such as acetic acid and hydrofluoric acid.
However, the terms "strong" and "weak" are really relative. pKa values that we have seen range from -5 to 50. If something with a pKa of 4 is described as a weak acid, what is something with a pKa of 25? A very, very weak acid? It is certainly a better source of protons than something with a pKa of 35. Is that a very, very, very, very weak acid? How many "verys" are there in a pKa unit?
This idea is also true when considering the opposite: a base picking up a proton to form a conjugate acid. How tightly that conjugate acid holds a proton is related to how strongly the base can remove protons from other acids. The weaker something is as a source of protons, the stronger its conjugate is as a proton sponge.
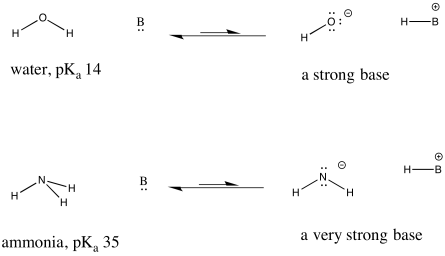
Figure AB9.6. Examples of a strong base and an even stronger one.
- Be careful. The terms "strong acid" and "weak acid" can be used relatively, rather than absolutely.
- The same is true for "strong base" and "weak base".
- Sometimes, whether something is called "strong" or "weak" depends on what else it is being compared to.
Table AB9.1. Approximate pKa values for selected compounds.
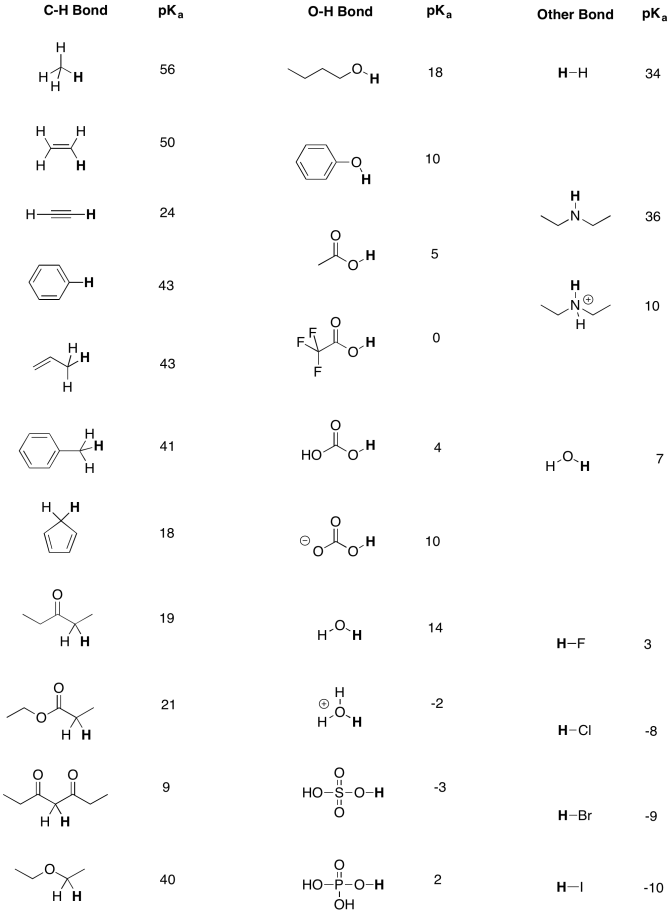
A more extensive pKa table can be found at Prof. David Evans' site at Harvard.
Problem AB9.1. Find a pKa table. Use it to help you decide which of the following pairs is the most Bronsted acidic in water.
a) HNO3 or HNO2 b) H2Se or H2O c) HCl or H2SO4 d) Be(OH)2 or HSeO3
Problem AB9.2. Find a pKa table. Use it to help you decide which of the compounds in each pair forms the most basic conjugate after deprotonation in water.
a) NH4+ or HN3 b) HCN or HSCN c) NH3 or H2O
* A note on the pKa of water: physics and physical chemistry texts list 14 as the value of the pKa of water. Biochemistry and organic chemistry texts often list the value as 15.7. The latter texts have simply factored into the constant a molar value for the concentration of water; the former agree that this factor should be replaced by the activity of water, which has a value of 1.
