Structure & Reactivity
Infrared Spectroscopy
IR6. Carbon-Oxygen Double Bonds
The largest class of oxygen-containing molecules is
carbonyl compounds, which contain C=O bonds. A C=O stretch is normally easy to
find in an IR spectrum, because it is very strong and shows up in a part of the
spectrum that isn't cluttered with other peaks. Examples of carbonyl compounds
include 2-octanone, a ketone, and butanal, an aldehyde. In an aldehyde, the
carbonyl is at the end of a chain, with a hydrogen attached to the carbonyl
carbon.
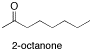
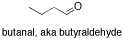
If you look at the IR spectrum of 2-octanone:
- there are sp3 C-H stretching and CH2 bending modes
at 2900 and 1500 cm-1.
- there is a very strong peak around 1700 cm-1.
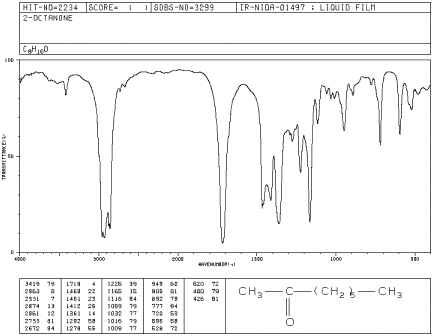
Figure IR6.1. IR spectrum of 2-octanone.
Even though there is just one C=O bond, the carbonyl
stretch is often the strongest peak in the spectrum. That makes carbonyl
compounds easy to identify by IR spectroscopy.
If you look at the IR spectrum of butanal:
- there are sp3 C-H stretching and CH2 bending modes
at 2900 and 1500 cm-1.
- there is a very strong C=O peak around 1700 cm-1.
- there is a pair of medium peaks around 2700 and 2800 cm-1.
This is the aldehyde C-H stretching mode.
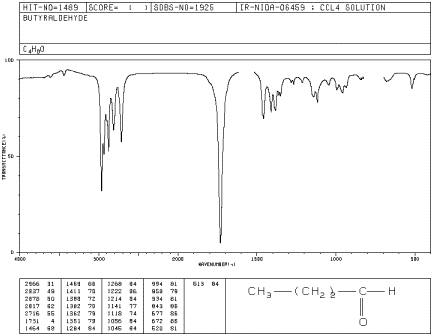
Figure IR6.2. IR spectrum of butanal.
The aldehyde C-H bond absorbs at two frequencies
because it can vibrate in phase with the C=O bond (a symmetric stretch) and out
of phase with the C=O bond (an asymmetric stretch), and these vibrations are of
different energies. The probability of the symmetric stretch and the asymmetric
stretch are about equal, so the two peaks are always about the same size. This
unusual C-H peak can often be used to distinguish between an aldehyde and a
ketone.
1. Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/
(National Institute of Advanced Industrial Science and Technology of Japan, 14
July 2008)
Back Next
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
University (with contributions from other authors as noted). It is freely
available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Infrared Spectroscopy
Back to Structure Determination
Back to Structure & Reactivity

![]()


